Inflammatory Bowel Disease Outcomes Following Fecal Microbiota Transplantation for Recurrent C. difficile Infection
- PMID: 33155639
- PMCID: PMC8376126
- DOI: 10.1093/ibd/izaa283
Inflammatory Bowel Disease Outcomes Following Fecal Microbiota Transplantation for Recurrent C. difficile Infection
Abstract
Background: Recurrent Clostridioides difficile infection (CDI) in patients with inflammatory bowel disease (IBD) is a clinical challenge. Fecal microbiota transplantation (FMT) has emerged as a recurrent CDI therapy. Anecdotal concerns exist regarding worsening of IBD activity; however, prospective data among IBD patients are limited.
Methods: Secondary analysis from an open-label, prospective, multicenter cohort study among IBD patients with 2 or more CDI episodes was performed. Participants underwent a single FMT by colonoscopy (250 mL, healthy universal donor). Secondary IBD-related outcomes included rate of de novo IBD flares, worsening IBD, and IBD improvement-all based on Mayo or Harvey-Bradshaw index (HBI) scores. Stool samples were collected for microbiome and targeted metabolomic profiling.
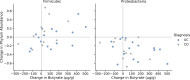
Results: Fifty patients enrolled in the study, among which 15 had Crohn's disease (mean HBI, 5.8 ± 3.4) and 35 had ulcerative colitis (mean partial Mayo score, 4.2 ± 2.1). Overall, 49 patients received treatment. Among the Crohn's disease cohort, 73.3% (11 of 15) had IBD improvement, and 4 (26.6%) had no disease activity change. Among the ulcerative colitis cohort, 62% (22 of 34) had IBD improvement, 29.4% (11 of 34) had no change, and 4% (1 of 34) experienced a de novo flare. Alpha diversity significantly increased post-FMT, and ulcerative colitis patients became more similar to the donor than Crohn's disease patients (P = 0.04).
Conclusion: This prospective trial assessing FMT in IBD-CDI patients suggests IBD outcomes are better than reported in retrospective studies.
Keywords: Clostridioides difficile infection; Crohn’s disease; butyrate; fecal microbiota transplantation; inflammatory bowel disease; microbiome; ulcerative colitis.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–455. - PubMed
-
- Zar FA, Bakkanagari SR, Moorthi KM, et al. . A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. - PubMed
-
- Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011;8:17–26. - PubMed
-
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

