Molecular targeting therapies for neuroblastoma: Progress and challenges
- PMID: 33155698
- PMCID: PMC7906923
- DOI: 10.1002/med.21750
Molecular targeting therapies for neuroblastoma: Progress and challenges
Erratum in
-
Corrigendum: Molecular targeting therapies for neuroblastoma: Progress and challenges.Med Res Rev. 2022 Jan;42(1):641. doi: 10.1002/med.21843. Epub 2021 Jul 11. Med Res Rev. 2022. PMID: 34250611 Free PMC article. No abstract available.
Abstract
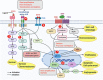
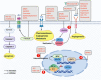
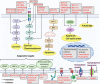
There is an urgent need to identify novel therapies for childhood cancers. Neuroblastoma is the most common pediatric solid tumor, and accounts for ~15% of childhood cancer-related mortality. Neuroblastomas exhibit genetic, morphological and clinical heterogeneity, which limits the efficacy of existing treatment modalities. Gaining detailed knowledge of the molecular signatures and genetic variations involved in the pathogenesis of neuroblastoma is necessary to develop safer and more effective treatments for this devastating disease. Recent studies with advanced high-throughput "omics" techniques have revealed numerous genetic/genomic alterations and dysfunctional pathways that drive the onset, growth, progression, and resistance of neuroblastoma to therapy. A variety of molecular signatures are being evaluated to better understand the disease, with many of them being used as targets to develop new treatments for neuroblastoma patients. In this review, we have summarized the contemporary understanding of the molecular pathways and genetic aberrations, such as those in MYCN, BIRC5, PHOX2B, and LIN28B, involved in the pathogenesis of neuroblastoma, and provide a comprehensive overview of the molecular targeted therapies under preclinical and clinical investigations, particularly those targeting ALK signaling, MDM2, PI3K/Akt/mTOR and RAS-MAPK pathways, as well as epigenetic regulators. We also give insights on the use of combination therapies involving novel agents that target various pathways. Further, we discuss the future directions that would help identify novel targets and therapeutics and improve the currently available therapies, enhancing the treatment outcomes and survival of patients with neuroblastoma.
Keywords: clinical; neuroblastoma; preclinical; signaling pathway; targeted therapy.
© 2020 The Authors. Medicinal Research Reviews published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
References
-
- Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999‐2007: results of EUROCARE‐5—a population‐based study. Lancet Oncol. 2014;15(1):35‐47. - PubMed
-
- Tonini GP, Capasso M. Genetic predisposition and chromosome instability in neuroblastoma. Cancer Metastasis Rev. 2020;39(1):275‐285. - PubMed
-
- Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

