Relative expression of microRNAs, apoptosis, and ultrastructure anomalies induced by gold nanoparticles in Trachyderma hispida (Coleoptera: Tenebrionidae)
- PMID: 33156883
- PMCID: PMC7647063
- DOI: 10.1371/journal.pone.0241837
Relative expression of microRNAs, apoptosis, and ultrastructure anomalies induced by gold nanoparticles in Trachyderma hispida (Coleoptera: Tenebrionidae)
Abstract
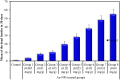
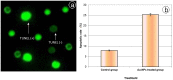
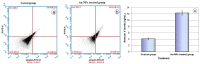
The extensive use of nanomaterials generates toxic effects on non-target species and the ecosystem. Although gold nanoparticles (Au-NPs) are generally expected to be safe, the recent study contains conflicting data regarding their cytotoxicity in the darkling beetles Trachyderma hispida. The study postulated cellular perturbation in the ovarian tissue of the beetles induced by a sublethal dose of Au-NPs (0.01 mg/g). When compared with the controls, a significant inhibition in the activities of the antioxidant enzymes selenium-dependent (GPOX) and selenium-independent (GSTP) glutathione peroxidases (GPx) was observed in the treated beetles. The study proposed microRNAs (miRNA-282 and miRNA-989) as genotoxic markers for the first time, reporting a significant suppression in their transcriptional levels in the treated beetles. Furthermore, TUNEL (Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) and flow cytometry assays (annexin V-Fitc) indicated a significant increase in ovarian cell apoptosis in the treated beetles. Additionally, an ultrastructure examination revealed pathological changes in the ovarian cells of the treated beetles. The resulting anomalies in the present study may interrupt the fecundity of the beetles and lead to the future suppression of beetle populations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

