4-methylpyrazole protects against acetaminophen-induced acute kidney injury
- PMID: 33157119
- PMCID: PMC7888547
- DOI: 10.1016/j.taap.2020.115317
4-methylpyrazole protects against acetaminophen-induced acute kidney injury
Abstract
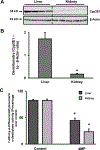
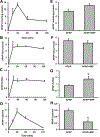
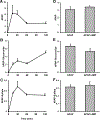
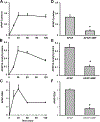
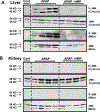
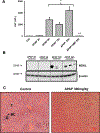
Acetaminophen (APAP) hepatotoxicity is the most common cause of acute liver failure in the United States, and while a significant percentage of APAP overdose patients develop kidney injury, molecular mechanisms involved in APAP-induced nephrotoxicity are relatively unknown. We have shown that 4-methylpyrazole (4MP, Fomepizole) protects against APAP-induced liver injury by inhibiting reactive metabolite formation through Cyp2E1, and analysis of data from APAP overdose patients indicated that kidney dysfunction strongly correlated with severe liver injury. Since Cyp2E1 is also expressed in the kidney, this study explored protection by 4MP against APAP-induced nephrotoxicity. Male C57BL/6 J mice were treated with either 300 or 600 mg/kg APAP with or without 4MP for 2, 6 or 24 h, followed by measurement of APAP metabolism and tissue injury. Interestingly, levels of APAP and its non-oxidative metabolites were significantly higher in kidneys when compared to the liver. APAP-protein adducts were present in both tissues within 2 h, but were absent in kidney mitochondria, unlike in the liver. While GSH depletion was seen in both tissues, activation of c-jun N-terminal kinase and its translocation to the mitochondria, which is a critical feature of APAP-induced liver injury, was not detected in the kidney. Treatment with 4MP attenuated APAP oxidative metabolite generation, GSH depletion as well as kidney injury indicating its potential use in protection against APAP-induced nephrotoxicity. In conclusion, since reactive metabolite formation seems to be common in both liver and kidney, 4MP mediated inhibition of Cyp2E1 protects against APAP-induced nephrotoxicity. However, downstream mechanisms of APAP-induced nephrotoxicity seem distinct from the liver.
Keywords: Acetaminophen; Fomepizole; Hepatotoxicity; N-Acetylcysteine; Nephrotoxicity; Protein Adducts.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures




References
-
- Arzuk E, Turna B, Sözbilen M, Orhan H, 2018. Inter-individual and inter-organ variability in the bioactivation of paracetamol by human liver and kidney tissues. Environ. Toxicol. Pharmacol 61, 8–17. - PubMed
-
- Björck S, Svalander CT, Aurell M, 1988. Acute renal failure after analgesic drugs including paracetamol (acetaminophen). Nephron 49, 45–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

