A thermoresponsive hydrogel system for long-acting corticosteroid delivery into the paranasal sinuses
- PMID: 33157189
- PMCID: PMC8444195
- DOI: 10.1016/j.jconrel.2020.10.062
A thermoresponsive hydrogel system for long-acting corticosteroid delivery into the paranasal sinuses
Abstract
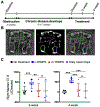
Delivering localized treatment to the paranasal sinuses for diseases such as chronic rhinosinusitis (CRS) is particularly challenging because of the small natural openings leading from the sinuses that can be further obstructed by presence of inflammation. As such, oral steroids, topical nasal sprays or irrigation, and surgery can be utilized to treat persistent sinonasal inflammation, but there exists a need for post-operative options for long-term steroid delivery to prevent disease recurrence. In the present study, a Thermogel, Extended-release Microsphere-based-delivery to the Paranasal Sinuses (TEMPS) is developed with the corticosteroid mometasone furoate. Specifically, the bioactive steroid is released for 4 weeks from poly(lactic-co-glycolic acid) (PLGA) microspheres embedded in a poly(N-isopropylacrylamide) (p-NIPAAm)-based hydrogel. The temperature-responsive system undergoes a reversible sol-gel transition at 34-35 °C such that it can be applied as a liquid at ambient temperature, conforming to the sinonasal epithelium as it gels. In a rabbit model of CRS, TEMPS was maintained in rabbit sinuses and effectively reduced sinonasal inflammation as characterized by micro-computed tomography and histopathology analysis. Ultimately, the combination of controlled release microspheres with a thermoresponsive hydrogel provides flexibility for encapsulating therapeutics in a reversible and conforming system for localized delivery to the sinuses.
Keywords: Hydrogel; Microsphere; Sinusitis; Steroid; Sustained release; Thermoresponsive.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
None.
Figures
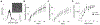
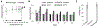
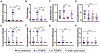

References
-
- Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, Orlandi RR, Palmer JN, Patel ZM, Peters A, Walsh SA, Corrigan MD, Clinical practice guideline (update): adult sinusitis, Otolaryngol. - Head Neck Surg. (United States) 152 (2015) S1–S39, 10.1177/0194599815572097. - DOI - PubMed
-
- Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, Batra PS, Bernal-Sprekelsen M, Bhattacharyya N, Chandra RK, Chiu A, Citardi MJ, Cohen NA, Delgaudio J, Desrosiers M, Dhong HJ, Douglas R, Ferguson B, Fokkens WJ, Georgalas C, Goldberg A, Gosepath J, Hamilos DL, Han JK, Harvey R, Hellings P, Hopkins C, Jankowski R, Javer AR, Kern R, Kountakis S, Kowalski ML, Lane A, Lanza DC, Lebowitz R, Lee HM, Lin SY, Lund V, Luong A, Mann W, Marple BF, Mcmains KC, Metson R, Naclerio R, Nayak JV, Otori N, Palmer JN, Parikh SR, Passali D, Peters A, Piccirillo J, Poetker DM, Psaltis AJ, Ramadan HH, Ramakrishnan VR, Riechelmann H, Roh HJ, Rudmik L, Sacks R, Schlosser RJ, Senior BA, Sindwani R, Stankiewicz JA, Stewart M, Tan BK, Toskala E, Voegels R, Wang DY, Weitzel EK, Wise S, Woodworth BA, Wormald PJ, Wright ED, Zhou B, Kennedy DW, International consensus statement on allergy and rhinology: rhinosinusitis, Int. Forum Allergy Rhinol 6 (2016) S22–S209, 10.1002/alr.21695. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

