Myelin as a regulator of development of the microbiota-gut-brain axis
- PMID: 33157256
- PMCID: PMC7749851
- DOI: 10.1016/j.bbi.2020.11.001
Myelin as a regulator of development of the microbiota-gut-brain axis
Abstract
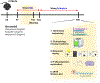
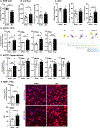
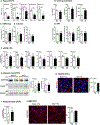
Myelination in the peripheral and central nervous systems is critical in regulating motor, sensory, and cognitive functions. As myelination occurs rapidly during early life, neonatal gut dysbiosis during early colonization can potentially alter proper myelination by dysregulating immune responses and neuronal differentiation. Despite common usage of antibiotics (Abx) in children, the impact of neonatal Abx-induced dysbiosis on the development of microbiota, gut, brain (MGB) axis, including myelination and behavior, is unknown. We hypothesized that neonatal Abx-induced dysbiosis dysregulates host-microbe interactions, impairing myelination in the brain, and altering the MGB axis. Neonatal C57BL/6 mice were orally gavaged daily with an Abx cocktail (neomycin, vancomycin, ampicillin) or water (vehicle) from postnatal day 7 (P7) until weaning (P23) to induce gut dysbiosis. Behavior (cognition; anxiety-like behavior), microbiota sequencing, and qPCR (ileum, colon, hippocampus and pre-frontal cortex [PFC]) were performed in adult mice (6-8 weeks). Neonatal Abx administration led to intestinal dysbiosis in adulthood, impaired intestinal physiology, coupled with perturbations of bacterial metabolites and behavioral alterations (cognitive deficits and anxiolytic behavior). Expression of myelin-related genes (Mag, Mog, Mbp, Mobp, Plp) and transcription factors (Sox10, Myrf) important for oligodendrocytes were significantly increased in the PFC region of Abx-treated mice. Increased myelination was confirmed by immunofluorescence imaging and western blot analysis, demonstrating increased expression of MBP, SOX10 and MYRF in neonatally Abx-treated mice compared to sham controls in adulthood. Finally, administration of the short chain fatty acid butyrate following completion of the Abx treatment restored intestinal physiology, behavior, and myelination impairments, suggesting a critical role for the gut microbiota in mediating these effects. Taken together, we identified a long-lasting impact of neonatal Abx administration on the MGB axis, specifically on myelin regulation in the PFC region, potentially contributing to impaired cognitive function and bacterial metabolites are effective in reversing this altered phenotype.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Letter to editor about the article "Myelin as a regulator of development of the microbiota-gut-brain axis".Brain Behav Immun. 2022 Feb;100:242. doi: 10.1016/j.bbi.2021.12.010. Epub 2021 Dec 15. Brain Behav Immun. 2022. PMID: 34920090 No abstract available.
-
In response to the letter by BOSTANCIKLIOGLU.Brain Behav Immun. 2022 Mar;101:59. doi: 10.1016/j.bbi.2021.12.011. Epub 2021 Dec 27. Brain Behav Immun. 2022. PMID: 34968717 No abstract available.
-
The role of Tregs on the butyrate-mediated demyelination and remyelination.Brain Behav Immun. 2022 Mar;101:180-181. doi: 10.1016/j.bbi.2022.01.012. Epub 2022 Jan 12. Brain Behav Immun. 2022. PMID: 35032572 No abstract available.
References
-
- Benes FM, 1989. Myelination of cortical-hippocampal relays during late adolescence. Schizophr. Bull 15, 585–593. - PubMed
-
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodriguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS 2nd, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vazquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG, 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol 37, 852–857. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

