A Review on New 3-D Printed Materials' Geometries for Catalysis and Adsorption: Paradigms from Reforming Reactions and CO2 Capture
- PMID: 33158048
- PMCID: PMC7693986
- DOI: 10.3390/nano10112198
A Review on New 3-D Printed Materials' Geometries for Catalysis and Adsorption: Paradigms from Reforming Reactions and CO2 Capture
Abstract
"Bottom-up" additive manufacturing (AM) is the technology whereby a digitally designed structure is built layer-by-layer, i.e., differently than by traditional manufacturing techniques based on subtractive manufacturing. AM, as exemplified by 3D printing, has gained significant importance for scientists, among others, in the fields of catalysis and separation. Undoubtedly, it constitutes an enabling pathway by which new complex, promising and innovative structures can be built. According to recent studies, 3D printing technologies have been utilized in enhancing the heat, mass transfer, adsorption capacity and surface area in CO2 adsorption and separation applications and catalytic reactions. However, intense work is needed in the field to address further challenges in dealing with the materials and metrological features of the structures involved. Although few studies have been performed, the promise is there for future research to decrease carbon emissions and footprint. This review provides an overview on how AM is linked to the chemistry of catalysis and separation with particular emphasis on reforming reactions and carbon adsorption and how efficient it could be in enhancing their performance.
Keywords: 3D printing; CO2 capture; additive manufacturing; adsorbents; carbon dioxide; catalysts; reforming.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















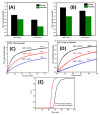




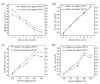

References
-
- Hannah Ritchie M.R. CO2 and Greenhouse Gas Emissions. [(accessed on 2 August 2020)];2017 Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions.
-
- Hertwich E. The Carbon Footprint of Material Production Rises to 23% of Global Greenhouse Gas Emissions. SocArXiv. 2019 doi: 10.31235/osf.io/n9ecw. - DOI
-
- Kontos A.G., Likodimos V., Veziri C.M., Kouvelos E., Moustakas N., Karanikolos G.N., Romanos G.E., Falaras P. CO2 Captured in Zeolitic Imidazolate Frameworks: Raman Spectroscopic Analysis of Uptake and Host-Guest Interactions. ChemSusChem. 2014;7:1696–1702. doi: 10.1002/cssc.201301323. - DOI - PubMed
-
- Department of the Environment and Heritage Sulfur Dioxide (SO2), Air Quality Fact Sheet. [(accessed on 2 August 2020)];2005 Available online: https://www.environment.gov.au/protection/publications/factsheet-sulfur-....
-
- Talapaneni S.N., Buyukcakir O., Je S.H., Srinivasan S., Seo Y., Polychronopoulou K., Coskun A. Nanoporous Polymers Incorporating Sterically Confined N-Heterocyclic Carbenes for Simultaneous CO2 Capture and Conversion at Ambient Pressure. Chem. Mater. 2015;27:6818–6826. doi: 10.1021/acs.chemmater.5b03104. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

