The Specific Effects of OD-1, a Peptide Activator, on Voltage-Gated Sodium Current and Seizure Susceptibility
- PMID: 33158049
- PMCID: PMC7663472
- DOI: 10.3390/ijms21218254
The Specific Effects of OD-1, a Peptide Activator, on Voltage-Gated Sodium Current and Seizure Susceptibility
Abstract
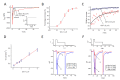
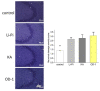
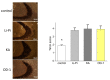
OD-1, a scorpion toxin, has been previously recognized as an activator of voltage-gated Na+ currents. To what extent this agent can alter hippocampal neuronal Na+ currents and network excitability and how it can be applied to neuronal hyperexcitability research remains unclear. With the aid of patch-clamp technology, it was revealed that, in mHippoE-14 hippocampal neurons, OD-1 produced a concentration-, time-, and state-dependent rise in the peak amplitude of INa. It shifted the INa inactivation curve to a less negative potential and increased the frequency of spontaneous action currents. Further characterization of neuronal excitability revealed higher excitability in the hippocampal slices treated with OD-1 as compared with the control slices. A stereotaxic intrahippocampal injection of OD-1 generated a significantly higher frequency of spontaneous seizures and epileptiform discharges compared with intraperitoneal injection of lithium-pilocarpine- or kainic acid-induced epilepsy, with comparable pathological changes. Carbamazepine significantly attenuated OD-1 induced seizures and epileptiform discharges. The OD-1-mediated modifications of INa altered the electrical activity of neurons in vivo and OD-1 could potentially serve as a novel seizure and excitotoxicity model.
Keywords: OD-1; action current; neuronal excitability; scorpion toxin; seizure; voltage-gated Na+ current.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Telmisartan, an Antagonist of Angiotensin II Receptors, Accentuates Voltage-Gated Na+ Currents and Hippocampal Neuronal Excitability.Front Neurosci. 2020 Sep 4;14:902. doi: 10.3389/fnins.2020.00902. eCollection 2020. Front Neurosci. 2020. PMID: 33013297 Free PMC article.
-
Sodium Metabisulfite: Effects on Ionic Currents and Excitotoxicity.Neurotox Res. 2018 Jul;34(1):1-15. doi: 10.1007/s12640-017-9844-4. Epub 2017 Nov 29. Neurotox Res. 2018. PMID: 29188487
-
The Novel Effect of Immunomodulator-Glatiramer Acetate on Epileptogenesis and Epileptic Seizures.Cell Physiol Biochem. 2018;50(1):150-168. doi: 10.1159/000493965. Epub 2018 Oct 2. Cell Physiol Biochem. 2018. PMID: 30278465
-
Characterizing the effects of Eugenol on neuronal ionic currents and hyperexcitability.Psychopharmacology (Berl). 2012 Jun;221(4):575-87. doi: 10.1007/s00213-011-2603-y. Epub 2011 Dec 13. Psychopharmacology (Berl). 2012. PMID: 22160139
-
The Protective Role of Peroxisome Proliferator-Activated Receptor-Gamma in Seizure and Neuronal Excitotoxicity.Mol Neurobiol. 2019 Aug;56(8):5497-5506. doi: 10.1007/s12035-018-1457-2. Epub 2019 Jan 8. Mol Neurobiol. 2019. PMID: 30623373
Cited by
-
The Integrated Effects of Brivaracetam, a Selective Analog of Levetiracetam, on Ionic Currents and Neuronal Excitability.Biomedicines. 2021 Apr 1;9(4):369. doi: 10.3390/biomedicines9040369. Biomedicines. 2021. PMID: 33916190 Free PMC article.
-
Electrophysiological evaluation of the effect of peptide toxins on voltage-gated ion channels: a scoping review on theoretical and methodological aspects with focus on the Central and South American experience.J Venom Anim Toxins Incl Trop Dis. 2024 Sep 2;30:e20230048. doi: 10.1590/1678-9199-JVATITD-2023-0048. eCollection 2024. J Venom Anim Toxins Incl Trop Dis. 2024. PMID: 39263598 Free PMC article.
-
The Discordance between Network Excitability and Cognitive Performance Following Vigabatrin Treatment during Epileptogenesis.Life (Basel). 2021 Nov 10;11(11):1213. doi: 10.3390/life11111213. Life (Basel). 2021. PMID: 34833089 Free PMC article.
-
Exploring the Pivotal Components Influencing the Side Effects Induced by an Analgesic-Antitumor Peptide from Scorpion Venom on Human Voltage-Gated Sodium Channels 1.4 and 1.5 through Computational Simulation.Toxins (Basel). 2022 Dec 31;15(1):33. doi: 10.3390/toxins15010033. Toxins (Basel). 2022. PMID: 36668853 Free PMC article.
-
The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action.Int J Mol Sci. 2021 Nov 17;22(22):12399. doi: 10.3390/ijms222212399. Int J Mol Sci. 2021. PMID: 34830281 Free PMC article.
References
-
- Jalali A., Bosmans F., Amininasab M., Clynen E., Cuypers E., Zaremirakabadi A., Sarbolouki M.N., Schoofs L., Vatanpour H., Tytgat J. OD1, the first toxin isolated from the venom of the scorpion Odonthobuthus doriae active on voltage-gated Na+ channels. FEBS Lett. 2005;579:4181–4186. doi: 10.1016/j.febslet.2005.06.052. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

