The Evolving Role of Caveolin-1: A Critical Regulator of Extracellular Vesicles
- PMID: 33158117
- PMCID: PMC7712126
- DOI: 10.3390/medsci8040046
The Evolving Role of Caveolin-1: A Critical Regulator of Extracellular Vesicles
Abstract
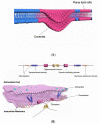
Emerging evidence suggests that extracellular vesicles (EVs) play an essential role in mediating intercellular communication and inter-organ crosstalk both at normal physiological conditions and in the pathogenesis of human diseases. EV cargos are made up of a broad spectrum of molecules including lipids, proteins, and nucleic acids such as DNA, RNA, and microRNAs. The complex EV cargo composition is cell type-specific. A dynamic change in EV cargos occurs along with extracellular stimuli and a change in the pathophysiological status of the host. Currently, the underlying mechanisms by which EVs are formed and EV cargos are selected in the absence and presence of noxious stimuli and pathogens remain incompletely explored. The term EVs refers to a heterogeneous group of vesicles generated via different mechanisms. Some EVs are formed via direct membrane budding, while the others are produced through multivesicular bodies (MVBs) or during apoptosis. Despite the complexity of EV formation and EV cargo selection, recent studies suggest that caveolin-1, a well-known structural protein of caveolae, regulates the formation and cargo selection of some EVs, such as microvesicles (MVs). In this article, we will review the current understanding of this emerging and novel role of cav-1.
Keywords: EV cargo; caveolin-1; extracellular vesicle (EVs).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Chargaff E., West R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946;166:189–197. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

