Umbilical Cord-Derived CD362+ Mesenchymal Stromal Cells Attenuate Polymicrobial Sepsis Induced by Caecal Ligation and Puncture
- PMID: 33158246
- PMCID: PMC7672591
- DOI: 10.3390/ijms21218270
Umbilical Cord-Derived CD362+ Mesenchymal Stromal Cells Attenuate Polymicrobial Sepsis Induced by Caecal Ligation and Puncture
Abstract
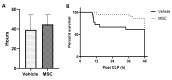
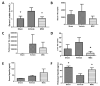
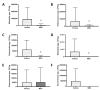
Mesenchymal stromal cells (MSCs) have a multimodal, immunomodulatory mechanism of action and are now in clinical trials for single organ and systemic sepsis. However, a number of practicalities around source, homogeneity and therapeutic window remain to be determined. Here, we utilised conditioned medium from CD362+-sorted umbilical cord-human MSCs (UC-hMSCs) for a series of in vitro anti-inflammatory assays and the cryopreserved MSCs themselves in a severe (Series 1) or moderate (Series 2+3) caecal ligation and puncture (CLP) rodent model. Surviving animals were assessed at 48 h post injury induction. MSCs improved human lung, colonic and kidney epithelial cell survival following cytokine activation. In severe systemic sepsis, MSCs administered at 30 min enhanced survival (Series 1), and reduced organ bacterial load. In moderate systemic sepsis (Series 2), MSCs were ineffective when delivered immediately or 24 h later. Of importance, MSCs delivered 4 h post induction of moderate sepsis (Series 3) were effective, improving serum lactate, enhancing bacterial clearance from tissues, reducing pro-inflammatory cytokine concentrations and increasing antimicrobial peptides in serum. While demonstrating benefit and immunomodulation in systemic sepsis, therapeutic efficacy may be limited to a specific point of disease onset, and repeat dosing, MSC enhancement or other contingencies may be necessary.
Keywords: inflammation; mesenchymal stem cell; sepsis.
Conflict of interest statement
Steve Elliman is the Chief Scientific Officer at Orbsen Therapeutics Ltd., Galway, Ireland, a company which is developing the Syndecan-2 positive mesenchymal stromal cells for therapeutic purposes. The other authors declare no competing interests.
Figures



Similar articles
-
Comparison of Single and Repeated Dosing of Anti-Inflammatory Human Umbilical Cord Mesenchymal Stromal Cells in a Mouse Model of Polymicrobial Sepsis.Stem Cell Rev Rep. 2022 Apr;18(4):1444-1460. doi: 10.1007/s12015-021-10323-7. Epub 2022 Jan 10. Stem Cell Rev Rep. 2022. PMID: 35013938 Free PMC article.
-
Umbilical cord-derived CD362+ mesenchymal stromal cells for E. coli pneumonia: impact of dose regimen, passage, cryopreservation, and antibiotic therapy.Stem Cell Res Ther. 2020 Mar 13;11(1):116. doi: 10.1186/s13287-020-01624-8. Stem Cell Res Ther. 2020. PMID: 32169108 Free PMC article.
-
Immunophenotypic characterization and therapeutics effects of human bone marrow- and umbilical cord-derived mesenchymal stromal cells in an experimental model of sepsis.Exp Cell Res. 2021 Feb 15;399(2):112473. doi: 10.1016/j.yexcr.2021.112473. Epub 2021 Jan 8. Exp Cell Res. 2021. PMID: 33428902
-
Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cell Therapy in Preclinical Models of Sepsis: A Systematic Review and Meta-analysis.Stem Cells Transl Med. 2024 Apr 15;13(4):346-361. doi: 10.1093/stcltm/szae003. Stem Cells Transl Med. 2024. PMID: 38381583 Free PMC article.
-
The application of umbilical cord-derived MSCs in cardiovascular diseases.J Cell Mol Med. 2021 Sep;25(17):8103-8114. doi: 10.1111/jcmm.16830. Epub 2021 Aug 11. J Cell Mol Med. 2021. PMID: 34378345 Free PMC article. Review.
Cited by
-
Comparison of Single and Repeated Dosing of Anti-Inflammatory Human Umbilical Cord Mesenchymal Stromal Cells in a Mouse Model of Polymicrobial Sepsis.Stem Cell Rev Rep. 2022 Apr;18(4):1444-1460. doi: 10.1007/s12015-021-10323-7. Epub 2022 Jan 10. Stem Cell Rev Rep. 2022. PMID: 35013938 Free PMC article.
-
Application progress of single-cell sequencing technology in mesenchymal stem cells research.Front Cell Dev Biol. 2024 Jan 9;11:1336482. doi: 10.3389/fcell.2023.1336482. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38264356 Free PMC article. Review.
-
Human liver stem cells and derived extracellular vesicles protect from sepsis-induced acute lung injury and restore bone marrow myelopoiesis in a murine model of sepsis.Intensive Care Med Exp. 2024 Dec 3;12(1):111. doi: 10.1186/s40635-024-00701-z. Intensive Care Med Exp. 2024. PMID: 39627601 Free PMC article.
-
Unveiling heterogeneity in MSCs: exploring marker-based strategies for defining MSC subpopulations.J Transl Med. 2024 May 15;22(1):459. doi: 10.1186/s12967-024-05294-5. J Transl Med. 2024. PMID: 38750573 Free PMC article. Review.
-
Safety and efficacy of human umbilical cord mesenchymal stem cells for the treatment of sepsis induced by pneumonia: study protocol for a single-centre, randomised single-blind parallel group trial.BMJ Open. 2022 Apr 4;12(4):e058444. doi: 10.1136/bmjopen-2021-058444. BMJ Open. 2022. PMID: 35379638 Free PMC article.
References
-
- Gotts J.E., Matthay M.A. Sepsis: Pathophysiology and clinical management. BMJ. 2016;353:i1585. - PubMed
-
- Angus D.C., van der Poll T. Severe sepsis and septic shock. N. Engl. J. Med. 2013;369:2063. - PubMed
-
- Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138–150. - PubMed
-
- Ho M.S., Mei S.H., Stewart D.J. The Immunomodulatory and Therapeutic Effects of Mesenchymal Stromal Cells for Acute Lung Injury and Sepsis. J. Cell. Physiol. 2015;230:2606–2617. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

