SIRT1 Activation Using CRISPR/dCas9 Promotes Regeneration of Human Corneal Endothelial Cells through Inhibiting Senescence
- PMID: 33158256
- PMCID: PMC7694272
- DOI: 10.3390/antiox9111085
SIRT1 Activation Using CRISPR/dCas9 Promotes Regeneration of Human Corneal Endothelial Cells through Inhibiting Senescence
Abstract
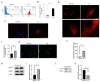
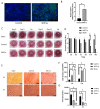
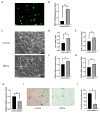
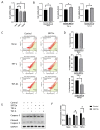
Human corneal endothelial cells (hCECs) are restricted in proliferative capacity in vivo. Reduction in the number of hCEC leads to persistent corneal edema requiring corneal transplantation. This study demonstrates the functions of SIRT1 in hCECs and its potential for corneal endothelial regeneration. Cell morphology, cell growth rates and proliferation-associated proteins were compared in normal and senescent hCECs. SIRT1 was activated using the CRISPR/dCas9 activation system (SIRT1a). The plasmids were transfected into CECs of six-week-old Sprague-Dawley rats using electroporation and cryoinjury was performed. Senescent cells were larger, elongated and showed lower proliferation rates and lower SIRT1 levels. SIRT1 activation promoted the wound healing of CECs. In vivo transfection of SIRT1a promoted the regeneration of CECs. The proportion of the S-phase cells was lower in senescent cells and elevated upon SIRT1a activation. SIRT1 regulated cell proliferation, proliferation-associated proteins, mitochondrial membrane potential, and oxidative stress levels. In conclusion, corneal endothelial senescence is related with a decreased SIRT1 level. SIRT1a promotes the regeneration of CECs by inhibiting cytokine-induced cell death and senescence. Gene function activation therapy using SIRT1a may serve as a novel treatment strategy for hCEC diseases.
Keywords: CRISPR/dCas9; SIRT1; corneal endothelial cells; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

