Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology
- PMID: 33158808
- PMCID: PMC7677597
- DOI: 10.1016/j.ebiom.2020.103104
Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology
Abstract
Background: COVID-19 is a deadly pulmonary disease with peculiar characteristics, which include variable clinical course and thrombophilia. A thorough understanding of the pathological correlates of the disease is still missing.
Methods: Here we report the systematic analysis of 41 consecutive post-mortem samples from individuals who died of COVID-19. Histological analysis is complemented by immunohistochemistry for cellular and viral antigens and the detection of viral genomes by in situ RNA hybridization.
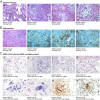
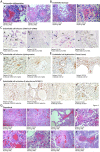
Findings: COVID-19 is characterized by extensive alveolar damage (41/41 of patients) and thrombosis of the lung micro- and macro-vasculature (29/41, 71%). Thrombi were in different stages of organization, consistent with their local origin. Pneumocytes and endothelial cells contained viral RNA even at the later stages of the disease. An additional feature was the common presence of a large number of dysmorphic pneumocytes, often forming syncytial elements (36/41, 87%). Despite occasional detection of virus-positive cells, no overt signs of viral infection were detected in other organs, which showed non-specific alterations.
Interpretation: COVID-19 is a unique disease characterized by extensive lung thrombosis, long-term persistence of viral RNA in pneumocytes and endothelial cells, along with the presence of infected cell syncytia. Several of COVID-19 features might be consequent to the persistence of virus-infected cells for the duration of the disease.
Funding: This work was supported by a King's Together Rapid COVID-19 Call grant from King's College London. MG is supported by the European Research Council (ERC) Advanced Grant 787971 "CuRE" and by Programme Grant RG/19/11/34633 from the British Heart Foundation.
Keywords: COVID-19; Endothelial dysfunction; Post-mortem analysis; SARS-CoV-2; Spike protein; Syncytia.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

