Magnetizing lead-free halide double perovskites
- PMID: 33158858
- PMCID: PMC7673701
- DOI: 10.1126/sciadv.abb5381
Magnetizing lead-free halide double perovskites
Abstract
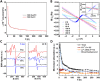
Spintronics holds great potential for next-generation high-speed and low-power consumption information technology. Recently, lead halide perovskites (LHPs), which have gained great success in optoelectronics, also show interesting magnetic properties. However, the spin-related properties in LHPs originate from the spin-orbit coupling of Pb, limiting further development of these materials in spintronics. Here, we demonstrate a new generation of halide perovskites, by alloying magnetic elements into optoelectronic double perovskites, which provide rich chemical and structural diversities to host different magnetic elements. In our iron-alloyed double perovskite, Cs2Ag(Bi:Fe)Br6, Fe3+ replaces Bi3+ and forms FeBr6 clusters that homogenously distribute throughout the double perovskite crystals. We observe a strong temperature-dependent magnetic response at temperatures below 30 K, which is tentatively attributed to a weak ferromagnetic or antiferromagnetic response from localized regions. We anticipate that this work will stimulate future efforts in exploring this simple yet efficient approach to develop new spintronic materials based on lead-free double perovskites.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Kojima A., Teshima K., Shirai Y., Miyasaka T., Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009). - PubMed
-
- Bai S., Da P., Li C., Wang Z., Yuan Z., Fu F., Kawecki M., Liu X., Sakai N., Wang J. T.-W., Huettner S., Buecheler S., Fahlman M., Gao F., Snaith H. J., Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 571, 245–250 (2019). - PubMed
-
- Wang N., Cheng L., Ge R., Zhang S., Miao Y., Zou W., Yi C., Sun Y., Cao Y., Yang R., Wei Y., Guo Q., Ke Y., Yu M., Jin Y., Liu Y., Ding Q., Di D., Yang L., Xing G., Tian H., Jin C., Gao F., Friend R. H., Wang J., Huang W., Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photonics 10, 699–704 (2016).
-
- Xu W., Hu Q., Bai S., Bao C., Miao Y., Yuan Z., Borzda T., Barker A. J., Tyukalova E., Hu Z., Kawecki M., Wang H., Yan Z., Liu X., Shi X., Uvdal K., Fahlman M., Zhang W., Duchamp M., Liu J.-M., Petrozza A., Wang J., Liu L.-M., Huang W., Gao F., Rational molecular passivation for high-performance perovskite light-emitting diodes. Nat. Photonics 13, 418–424 (2019).
-
- Zhu H., Fu Y., Meng F., Wu X., Gong Z., Ding Q., Gustafsson M. V., Trinh M. T., Jin S., Zhu X.-Y., Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 14, 636–642 (2015). - PubMed
LinkOut - more resources
Full Text Sources

