18F-AraG PET for CD8 Profiling of Tumors and Assessment of Immunomodulation by Chemotherapy
- PMID: 33158906
- PMCID: PMC8729865
- DOI: 10.2967/jnumed.120.249078
18F-AraG PET for CD8 Profiling of Tumors and Assessment of Immunomodulation by Chemotherapy
Abstract
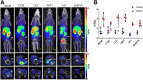
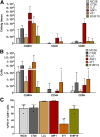
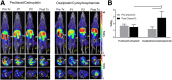
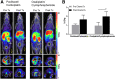
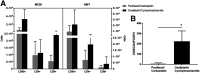
Most clinical trials exploring various combinations of chemo- and immunotherapy rely on serial biopsy to provide information on immune response. The aim of this study was to assess the value of 18F-arabinosyl guanine (18F-AraG) as a noninvasive tool that profiles tumors on the basis of the key player in adaptive antitumor response, CD8+ cells, and evaluates the immunomodulatory effects of chemotherapy. Methods: To evaluate the ability of 18F-AraG to report on the presence of CD8+ cells within the tumor microenvironment, we imaged a panel of syngeneic tumor models (MC38, CT26, LLC, A9F1, 4T1, and B16F10) and correlated the signal intensity with the number of lymphocytes found in the tumors. The capacity of 18F-AraG to detect immunomodulatory effects of chemotherapy was determined by longitudinal imaging of tumor-bearing mice (MC38 and A9F1) undergoing 2 types of chemotherapy: oxaliplatin/cyclophosphamide, shown to induce immunogenic cell death, and paclitaxel/carboplatin, reported to cause immunogenically silent tumor cell death. Results: In the tumor panel, 18F-AraG revealed strikingly different uptake patterns resembling cancer-immune phenotypes observed in the clinic. A statistically significant correlation was found between the 18F-AraG signal and the number of PD-1-positive CD8+ cells isolated from the tumors (r2 = 0.528, P < 0.0001). In the MC38 model, paclitaxel/carboplatin did not result in an appreciable change in signal after therapy (1.69 ± 0.25 vs. 1.50 ± 0.33 percentage injected dose per gram), but oxaliplatin/cyclophosphamide treatment led to close to a 2.4-fold higher 18F-AraG signal (1.20 ± 0.31 vs. 2.84 ± 0.93 percentage injected dose per gram). The statistically significant increase in signal after oxaliplatin/cyclophosphamide was also observed in the A9F1 model (0.95 ± 0.36 vs. 1.99 ± 0.54 percentage injected dose per gram). Conclusion: The ability of 18F-AraG PET to assess the location and function of CD8+ cells, as well immune activity within tumors after immune priming therapy, warrants further investigation into its utility for patient selection, evaluation of optimal time to deliver immunotherapies, and assessment of combinatorial therapies.
Keywords: CD8 T cells; chemotherapy; immunomodulation.
© 2021 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
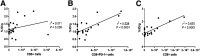
References
-
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. - PubMed
-
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. - PubMed
-
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
