Sub-nanoliter metabolomics via mass spectrometry to characterize volume-limited samples
- PMID: 33159052
- PMCID: PMC7648103
- DOI: 10.1038/s41467-020-19444-y
Sub-nanoliter metabolomics via mass spectrometry to characterize volume-limited samples
Abstract
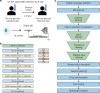
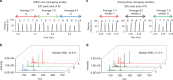
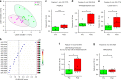
The human metabolome provides a window into the mechanisms and biomarkers of various diseases. However, because of limited availability, many sample types are still difficult to study by metabolomic analyses. Here, we present a mass spectrometry (MS)-based metabolomics strategy that only consumes sub-nanoliter sample volumes. The approach consists of combining a customized metabolomics workflow with a pulsed MS ion generation method, known as triboelectric nanogenerator inductive nanoelectrospray ionization (TENGi nanoESI) MS. Samples tested with this approach include exhaled breath condensate collected from cystic fibrosis patients as well as in vitro-cultured human mesenchymal stromal cells. Both test samples are only available in minimum amounts. Experiments show that picoliter-volume spray pulses suffice to generate high-quality spectral fingerprints, which increase the information density produced per unit sample volume. This TENGi nanoESI strategy has the potential to fill in the gap in metabolomics where liquid chromatography-MS-based analyses cannot be applied. Our method opens up avenues for future investigations into understanding metabolic changes caused by diseases or external stimuli.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Antonucci R, Atzori L, Barberini L, Fanos V. Metabolomics: the new clinical chemistry for personalized neonatal medicine. Minerva Pediatr. 2010;62:145–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

