Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin
- PMID: 33159076
- PMCID: PMC7648088
- DOI: 10.1038/s41467-020-19425-1
Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin
Abstract
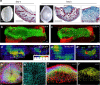
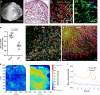
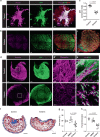
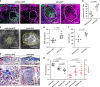
Scars are more severe when the subcutaneous fascia beneath the dermis is injured upon surgical or traumatic wounding. Here, we present a detailed analysis of fascia cell mobilisation by using deep tissue intravital live imaging of acute surgical wounds, fibroblast lineage-specific transgenic mice, and skin-fascia explants (scar-like tissue in a dish - SCAD). We observe that injury triggers a swarming-like collective cell migration of fascia fibroblasts that progressively contracts the skin and form scars. Swarming is exclusive to fascia fibroblasts, and requires the upregulation of N-cadherin. Both swarming and N-cadherin expression are absent from fibroblasts in the upper skin layers and the oral mucosa, tissues that repair wounds with minimal scar. Impeding N-cadherin binding inhibits swarming and skin contraction, and leads to reduced scarring in SCADs and in animals. Fibroblast swarming and N-cadherin thus provide therapeutic avenues to curtail fascia mobilisation and pathological fibrotic responses across a range of medical settings.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Sund, B. New Developments In Wound Care. 1–255 (London, PJB Publications, 2000). Clinical Report CBS 836.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

