Therapeutic Options in Hereditary Optic Neuropathies
- PMID: 33159657
- PMCID: PMC7843467
- DOI: 10.1007/s40265-020-01428-3
Therapeutic Options in Hereditary Optic Neuropathies
Abstract
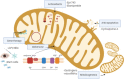
Options for the effective treatment of hereditary optic neuropathies have been a long time coming. The successful launch of the antioxidant idebenone for Leber's Hereditary Optic Neuropathy (LHON), followed by its introduction into clinical practice across Europe, was an important step forward. Nevertheless, other options, especially for a variety of mitochondrial optic neuropathies such as dominant optic atrophy (DOA), are needed, and a number of pharmaceutical agents, acting on different molecular pathways, are currently under development. These include gene therapy, which has reached Phase III development for LHON, but is expected to be developed also for DOA, whilst most of the other agents (other antioxidants, anti-apoptotic drugs, activators of mitobiogenesis, etc.) are almost all at Phase II or at preclinical stage of research. Here, we review proposed target mechanisms, preclinical evidence, available clinical trials with primary endpoints and results, of a wide range of tested molecules, to give an overview of the field, also providing the landscape of future scenarios, including gene therapy, gene editing, and reproductive options to prevent transmission of mitochondrial DNA mutations.
Conflict of interest statement
VC acts as scientific consultant in boards of GenSight Biologics, Stealth BioTherapeutics, Santhera Pharmaceuticals, and Chiesi. VC received speaker honoraria from Chiesi and an unrestricted research grant from Stealth BioTherapeutics. PB, MC, VC, CLM, MR, received speaker’s honoraria and travel support from Santhera Pharmaceuticals. PB received speaker honoraria from GenSight Biologics, Visufarma and Omkron. CLM received consulting fees from Regulatory Pharmanet and Chiesi Pharmaceuticals. GA, PB, MC, VC, CLM are investigators in clinical trials from Santhera Pharmaceuticals and GenSight Biologics. MR serves as Study Coordinator in Santhera Pharmaceuticals and GenSight Biologics sponsored trials. None of these activities are in any way related to the writing of this study.
Figures
References
-
- Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23(1):53–89. - PubMed
-
- Del Dotto V, Fogazza M, Carelli V, Rugolo M, Zanna C. Eight human opa1 isoforms, long and short: What are they for? Biochim Biophys Acta Bioenergy. 2018;1859(4):263–269. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources