Variation between rice accessions in photosynthetic induction in flag leaves and underlying mechanisms
- PMID: 33159790
- PMCID: PMC7904153
- DOI: 10.1093/jxb/eraa520
Variation between rice accessions in photosynthetic induction in flag leaves and underlying mechanisms
Abstract
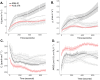
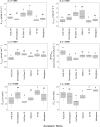
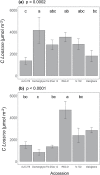
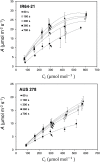
Several breeding initiatives have sought to improve flag leaf performance as its health and physiology are closely correlated to rice yield. Previous studies have described natural variation of photosynthesis for flag leaves; however, none has examined their performance under the non-steady-state conditions that prevail in crop fields. Photosynthetic induction is the transient response of photosynthesis to a change from low to high light. Rice flag leaf photosynthesis was measured in both steady- and non-steady-state conditions to characterize natural variation. Between the lowest and highest performing accession, there was a 152% difference for average CO2 assimilation during induction (Ā300), a 77% difference for average intrinsic water use efficiency during induction (iWUEavg), and a 185% difference for the speed of induction (IT50), indicating plentiful variation. No significant correlation was found between steady- and non-steady-state photosynthetic traits. Additionally, measures of neither steady-state nor non-steady-state photosynthesis of flag leaves correlated with the same measures of leaves in the vegetative growth stage, with the exception of iWUEavg. Photosynthetic induction was measured at six [CO2], to determine biochemical and diffusive limitations to photosynthesis in vivo. Photosynthetic induction in rice flag leaves was limited primarily by biochemistry.
Keywords: Atmospheric change; Rubisco activation; crop improvement; flag leaves; food security; natural variation; photosynthetic induction; rice; rice breeding; water use efficiency.
© Society for Experimental Biology 2020.
Figures



References
-
- Abou-Khalifa ABA, Misra N, El-Azeem A, Salem KM. 2008. Effect of leaf cutting on physiological traits and yield of two rice cultivars. African Journal of Plant Science 2, 147–150.
-
- Adachi S, Yoshikawa K, Yamanouchi U, Tanabata T, Sun J, Ookawa T, Yamamoto T, Sage RF, Hirasawa T, Yonemaru J. 2017. Fine mapping of Carbon Assimilation Rate 8, a quantitative trait locus for flag leaf nitrogen content, stomatal conductance and photosynthesis in rice. Frontiers in Plant Science 8, 60. - PMC - PubMed
-
- Bansal KC, Uprety DC, Abrol YP. 1993. Genetic variation in photosynthetic characteristics in wheat: causes and consequences. In: Abrol YP, Mohanty P, Govindjee, eds. Photosynthesis: photoreactions to plant productivity. Dordrecht: Springer Science + Business Media, 527–547.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

