Targeted Activation of Hippocampal Place Cells Drives Memory-Guided Spatial Behavior
- PMID: 33159859
- PMCID: PMC7754708
- DOI: 10.1016/j.cell.2020.09.061
Targeted Activation of Hippocampal Place Cells Drives Memory-Guided Spatial Behavior
Erratum in
-
Targeted Activation of Hippocampal Place Cells Drives Memory-Guided Spatial Behavior.Cell. 2020 Dec 23;183(7):2041-2042. doi: 10.1016/j.cell.2020.12.010. Cell. 2020. PMID: 33357402 Free PMC article. No abstract available.
Abstract
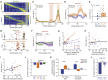
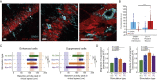
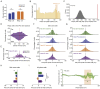
The hippocampus is crucial for spatial navigation and episodic memory formation. Hippocampal place cells exhibit spatially selective activity within an environment and have been proposed to form the neural basis of a cognitive map of space that supports these mnemonic functions. However, the direct influence of place cell activity on spatial navigation behavior has not yet been demonstrated. Using an 'all-optical' combination of simultaneous two-photon calcium imaging and two-photon optogenetics, we identified and selectively activated place cells that encoded behaviorally relevant locations in a virtual reality environment. Targeted stimulation of a small number of place cells was sufficient to bias the behavior of animals during a spatial memory task, providing causal evidence that hippocampal place cells actively support spatial navigation and memory.
Keywords: all-optical interrogation; behavior; hippocampus; inhibition; memory; place cell; spatial navigation; two-photon calcium imaging; two-photon optogenetics; virtual reality.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





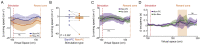


Comment in
-
Making the case for place cells.Nat Rev Neurosci. 2021 Jan;22(1):3. doi: 10.1038/s41583-020-00414-8. Nat Rev Neurosci. 2021. PMID: 33219370 No abstract available.
-
Spatial memory: Place cell activity is causally related to behavior.Curr Biol. 2021 Apr 12;31(7):R335-R337. doi: 10.1016/j.cub.2021.01.098. Curr Biol. 2021. PMID: 33848486 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

