A Clinic Blueprint for Post-Coronavirus Disease 2019 RECOVERY: Learning From the Past, Looking to the Future
- PMID: 33159907
- PMCID: PMC7641526
- DOI: 10.1016/j.chest.2020.10.067
A Clinic Blueprint for Post-Coronavirus Disease 2019 RECOVERY: Learning From the Past, Looking to the Future
Abstract
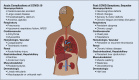
The severe acute respiratory syndrome coronavirus 2 pandemic poses extraordinary challenges. The tremendous number of coronavirus disease 2019 (COVID-19) cases in the United States has resulted in a large population of survivors with prolonged postinfection symptoms. The creation of multidisciplinary post-COVID-19 clinics to address both persistent symptoms and potential long-term complications requires an understanding of the acute disease and the emerging data regarding COVID-19 outcomes. Experience with severe acute respiratory syndrome and Middle East respiratory syndrome, post-acute respiratory distress syndrome complications, and post-intensive care syndrome also informs anticipated sequelae and clinical program design. Post-COVID-19 clinical programs should be prepared to care for individuals previously hospitalized with COVID-19 (including those who required critical care support), nonhospitalized individuals with persistent respiratory symptoms following COVID-19, and individuals with preexisting lung disease complicated by COVID-19. Effective multidisciplinary collaboration models leverage lessons learned during the early phases of the pandemic to overcome the unique logistical challenges posed by pandemic circumstances. Collaboration between physicians and researchers across disciplines will provide insight into survivorship that may shape the treatment of both acute disease and chronic complications. In this review, we discuss the aims, general principles, elements of design, and challenges of a successful multidisciplinary model to address the needs of COVID-19 survivors.
Keywords: COVID-19; SARS-CoV-2; clinical outcomes; multidisciplinary clinic design; post-COVID-19 programs.
Copyright © 2020 American College of Chest Physicians. All rights reserved.
Figures

References
-
- World Health Organization (WHO) Coronavirus Disease (COVID-19): Situation Report—138. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Centers for Disease Control and Prevention (CDC) CDC COVID Data Tracker: United States COVID-19 Cases and Deaths by State. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
-
- Connecticut Department of Public Health Connecticut COVID-19 Data Tracker. https://portal.ct.gov/Coronavirus
-
- Centers for Disease Control and Prevention (CDC) Coronavirus Disease (COVID-19): Health Equity Considerations and Racial and Ethnic Minority Groups. http://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-e...
Publication types
MeSH terms
Supplementary concepts
Grants and funding
- I01 BX004661/BX/BLRD VA/United States
- U24 HL138998/HL/NHLBI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 HL109233/HL/NHLBI NIH HHS/United States
- R01 OH012580/OH/NIOSH CDC HHS/United States
- UM1 AI114271/AI/NIAID NIH HHS/United States
- K76 AG057023/AG/NIA NIH HHS/United States
- R01 HL125850/HL/NHLBI NIH HHS/United States
- K08 HL151970/HL/NHLBI NIH HHS/United States
- R01 HL125897/HL/NHLBI NIH HHS/United States
- K23 HL138229/HL/NHLBI NIH HHS/United States
- U01 HL112702/HL/NHLBI NIH HHS/United States
- K24 HL132093/HL/NHLBI NIH HHS/United States
- P30 AG021342/AG/NIA NIH HHS/United States
- R01 HL126094/HL/NHLBI NIH HHS/United States
- UH2 HL123876/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

