Kidney epithelial targeted mitochondrial transcription factor A deficiency results in progressive mitochondrial depletion associated with severe cystic disease
- PMID: 33159962
- PMCID: PMC8209657
- DOI: 10.1016/j.kint.2020.10.013
Kidney epithelial targeted mitochondrial transcription factor A deficiency results in progressive mitochondrial depletion associated with severe cystic disease
Abstract
Abnormal mitochondrial function is a well-recognized feature of acute and chronic kidney diseases. To gain insight into the role of mitochondria in kidney homeostasis and pathogenesis, we targeted mitochondrial transcription factor A (TFAM), a protein required for mitochondrial DNA replication and transcription that plays a critical part in the maintenance of mitochondrial mass and function. To examine the consequences of disrupted mitochondrial function in kidney epithelial cells, we inactivated TFAM in sine oculis-related homeobox 2-expressing kidney progenitor cells. TFAM deficiency resulted in significantly decreased mitochondrial gene expression, mitochondrial depletion, inhibition of nephron maturation and the development of severe postnatal cystic disease, which resulted in premature death. This was associated with abnormal mitochondrial morphology, a reduction in oxygen consumption and increased glycolytic flux. Furthermore, we found that TFAM expression was reduced in murine and human polycystic kidneys, which was accompanied by mitochondrial depletion. Thus, our data suggest that dysregulation of TFAM expression and mitochondrial depletion are molecular features of kidney cystic disease that may contribute to its pathogenesis.
Keywords: TFAM; glycolysis; kidney development; mitochondria; polycystic kidney disease.
Copyright © 2020 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
VHH conceived the project. KI, HK and VHH designed the research studies, analyzed and interpreted data, wrote the manuscript and made figures. KI, HK, NG, KT, AL, CT, OL and CRB performed experiments, acquired and analyzed data. MS, NSC, and PVT provided mouse reagents and mouse tissues, conceptual input and assisted in the interpretation of data. ABF and MEK provided human tissues. The authors declare that no conflict of interest exists.
Figures
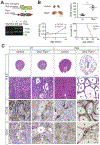
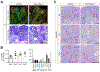
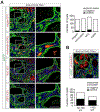
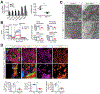
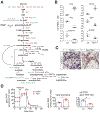
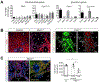
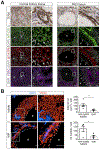
References
-
- Chandel NS. Evolution of Mitochondria as Signaling Organelles. Cell Metab 2015; 22: 204–206. - PubMed
-
- Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta 2012; 1819: 921–929. - PubMed
-
- Kukat C, Larsson NG. mtDNA makes a U-turn for the mitochondrial nucleoid. Trends Cell Biol 2013; 23: 457–463. - PubMed
-
- Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta 1999; 1410: 103–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK081646/DK/NIDDK NIH HHS/United States
- I01 BX002348/BX/BLRD VA/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 DK101791/DK/NIDDK NIH HHS/United States
- R01 DK108433/DK/NIDDK NIH HHS/United States
- P30 DK114809/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- I01 BX000320/BX/BLRD VA/United States
- P30 DK079341/DK/NIDDK NIH HHS/United States
- S10 OD023475/OD/NIH HHS/United States
- R01 DK056942/DK/NIDDK NIH HHS/United States
- R01 DK103033/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

