Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity
- PMID: 33160207
- PMCID: PMC7640894
- DOI: 10.1016/j.ebiom.2020.103101
Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity
Abstract
Background: Reliable high-throughput serological assays for SARS-CoV-2 antibodies are urgently needed for the effective containment of the COVID-19 pandemic, as it is of crucial importance to understand the strength and duration of immunity after infection, and to make informed decisions concerning the activation or discontinuation of physical distancing restrictions.
Methods: In 184 serum samples from 130 COVID-19 patients and 54 SARS-CoV-2 negative subjects, the analytical and clinical performances of four commercially available chemiluminescent assays (Abbott SARS-Cov-2 IgG, Roche Elecsys anti-SARS-CoV-2, Ortho SARS-CoV-2 total and IgG) and one enzyme-linked immunosorbent assay (Diesse ENZY-WELL SARS-CoV-2 IgG) were evaluated and compared with the neutralization activity achieved using the plaque reduction neutralization test (PRNT).
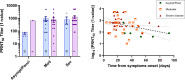
Findings: Precision results ranged from 0.9% to 11.8% for all assays. Elecsys anti-SARS-CoV-2 demonstrated linearity of results at concentrations within the cut-off value. Overall, sensitivity ranged from 78.5 to 87.7%, and specificity, from 97.6 to 100%. On limiting the analysis to samples collected 12 days after onset of symptoms, the sensitivity of all assays increased, the highest value (95.2%) being obtained with VITRO Anti-SARS-CoV-2 Total and Architect SARS-CoV-2 IgG. The strongest PRNT50 correlation with antibody levels was obtained with ENZY-Well SARS-CoV-2 IgG (R2adj = 0.569).
Interpretation: The results confirmed that all immunoassays had an excellent specificity, whereas sensitivity varied across immunoassays, depending strongly on the time interval between symptoms onset and sample collection. Further studies should be conducted to achieve a stronger correlation between antibody measurement and PRNT50 in order to obtain useful information for providing a better management of COVID-19 patients, effective passive antibody therapy, and developing a vaccine against the SARS-CoV-2 virus.
Funding: None.
Keywords: Antibodies; Clinical performances; Immunoassays; SARS-CoV-2; Serology.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Timeline of WHO's response to COVID-19. [updated 30 June 2020] Available from: https://www.who.int/news-room/detail/29-06-2020-covidtimeline.
-
- Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin Chem Lab Med. 2020;58:1070–1076. - PubMed
-
- Padoan A, Cosma C, Sciacovelli L, Faggian D, Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin Chem Lab Med. 2020;58:1081–1088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

