HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway
- PMID: 33160389
- PMCID: PMC7648939
- DOI: 10.1186/s13045-020-00985-0
HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway
Abstract
Background: Pyroptosis is a form of proinflammatory gasdermin-mediated programmed cell death. Abnormal mucosal inflammation in the intestine is a critical risk factor for colitis-associated colorectal cancer (CAC). However, it is unknown whether pyroptosis participates in the development of CAC.
Methods: To investigate the role of gasdermin E (GSDME)-mediated pyroptosis in the development of CAC, Gsdme-/- mice and their wild-type (WT) littermate controls were challenged with azoxymethane (AOM) and dextran sodium sulfate (DSS) to induce a CAC model. Neutralizing antibodies against high-mobility group box protein 1 (HMGB1) were used to determine the role of HMGB1 in CAC. To identify the role of ERK1/2 in HMGB1-induced colon cancer cell proliferation, we performed western blotting and CCK8 assays using the ERK1/2-specific inhibitor U0126 in CT26 colon cancer cells.
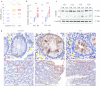
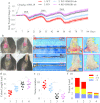
Results: In the CAC model, Gsdme-/- mice exhibited reduced weight loss and colon shortening, attenuated rectal prolapse, and reduced tumor numbers and sizes compared to WT littermates. Furthermore, treatment with neutralizing anti-HMGB1 antibodies decreased the numbers and sizes of tumors, ERK1/2 activation and proliferating cell nuclear antigen (PCNA) expression in AOM/DSS-challenged WT mice. In addition, our in vitro experiments demonstrated that HMGB1 induced proliferation and PCNA expression in CT26 colon cancer cells through the ERK1/2 pathway.
Conclusion: GSDME-mediated pyroptosis promotes the development of CAC by releasing HMGB1, which induces tumor cell proliferation and PCNA expression through the ERK1/2 pathway. This finding reveals a previously unrecognized link between pyroptosis and CAC tumorigenesis and offers new insight into CAC pathogenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Zhou CB, Fang JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection. Biochim Biophys Acta. 2019;1872:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

