Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2
- PMID: 33160446
- PMCID: PMC7604136
- DOI: 10.1016/j.cell.2020.10.043
Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2
Abstract
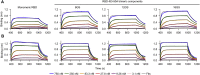
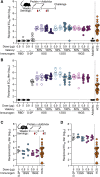
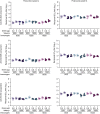
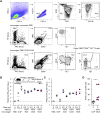
A safe, effective, and scalable vaccine is needed to halt the ongoing SARS-CoV-2 pandemic. We describe the structure-based design of self-assembling protein nanoparticle immunogens that elicit potent and protective antibody responses against SARS-CoV-2 in mice. The nanoparticle vaccines display 60 SARS-CoV-2 spike receptor-binding domains (RBDs) in a highly immunogenic array and induce neutralizing antibody titers 10-fold higher than the prefusion-stabilized spike despite a 5-fold lower dose. Antibodies elicited by the RBD nanoparticles target multiple distinct epitopes, suggesting they may not be easily susceptible to escape mutations, and exhibit a lower binding:neutralizing ratio than convalescent human sera, which may minimize the risk of vaccine-associated enhanced respiratory disease. The high yield and stability of the assembled nanoparticles suggest that manufacture of the nanoparticle vaccines will be highly scalable. These results highlight the utility of robust antigen display platforms and have launched cGMP manufacturing efforts to advance the SARS-CoV-2-RBD nanoparticle vaccine into the clinic.
Keywords: RBD; SARS-CoV-2; computational protein design; nanoparticle; protein; vaccine.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A.C.W, D.V., and N.P.K. are named as inventors on patent applications filed by the University of Washington based on the studies presented in this paper. N.P.K. is a co-founder, shareholder, paid consultant, and chair of the scientific advisory board of Icosavax, Inc. and has received an unrelated sponsored research agreement from Pfizer. D.V. is a consultant for and has received an unrelated sponsored research agreement from Vir Biotechnology Inc. H.Y.C. is a consultant for Merck and Pfizer and has received research funding from Sanofi-Pasteur, Roche-Genentech, Cepheid, and Ellume outside of the submitted work. P.K., A.P., and S.C. are employees and shareholders of Kymab Ltd. The other authors declare no competing interests.
Figures









Update of
-
Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2.bioRxiv [Preprint]. 2020 Aug 12:2020.08.11.247395. doi: 10.1101/2020.08.11.247395. bioRxiv. 2020. Update in: Cell. 2020 Nov 25;183(5):1367-1382.e17. doi: 10.1016/j.cell.2020.10.043. PMID: 32817941 Free PMC article. Updated. Preprint.
References
-
- Anywaine Z., Whitworth H., Kaleebu P., Praygod G., Shukarev G., Manno D., Kapiga S., Grosskurth H., Kalluvya S., Bockstal V. Safety and Immunogenicity of a 2-Dose Heterologous Vaccination Regimen With Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-Month Data From a Phase 1 Randomized Clinical Trial in Uganda and Tanzania. J. Infect. Dis. 2019;220:46–56. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

