Selective cysteines oxidation in soluble guanylyl cyclase catalytic domain is involved in NO activation
- PMID: 33161042
- PMCID: PMC7889651
- DOI: 10.1016/j.freeradbiomed.2020.11.001
Selective cysteines oxidation in soluble guanylyl cyclase catalytic domain is involved in NO activation
Abstract
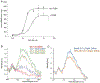
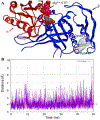
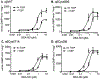
Nitric oxide (NO) binds to soluble guanylyl cyclase (GC1) and stimulates its catalytic activity to produce cGMP. Despite the key role of the NO-cGMP signaling in cardiovascular physiology, the mechanisms of GC1 activation remain ill-defined. It is believed that conserved cysteines (Cys) in GC1 modulate the enzyme's activity through thiol-redox modifications. We previously showed that GC1 activity is modulated via mixed-disulfide bond by protein disulfide isomerase and thioredoxin 1. Herein we investigated the novel concept that NO-stimulated GC1 activity is mediated by thiol/disulfide switches and aimed to map the specific Cys that are involved. First, we showed that the dithiol reducing agent Tris (2-carboxyethyl)-phosphine reduces GC1 response to NO, indicating the significance of Cys oxidation in NO activation. Second, using dibromobimane, which fluoresces when crosslinking two vicinal Cys thiols, we demonstrated decreased fluorescence in NO-stimulated GC1 compared to unstimulated conditions. This suggested that NO-stimulated GC1 contained more bound Cys, potentially disulfide bonds. Third, to identify NO-regulated Cys oxidation using mass spectrometry, we compared the redox status of all Cys identified in tryptic peptides, among which, ten were oxidized and two were reduced in NO-stimulated GC1. Fourth, we resorted to computational modeling to narrow down the Cys candidates potentially involved in disulfide bond and identified Cys489 and Cys571. Fifth, our mutational studies showed that Cys489 and Cys571 were involved in GC1'response to NO, potentially as a thiol/disulfide switch. These findings imply that specific GC1 Cys sensitivity to redox environment is critical for NO signaling in cardiovascular physiology.
Keywords: Allosteric regulation; Crosslinking; Cyclic GMP (cGMP); Disulfide; Nitric oxide (NO); Soluble guanylyl cyclase (GC1); Thiol.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures
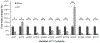


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

