Lysosomal dysfunction in osteoarthritis and aged cartilage triggers apoptosis in chondrocytes through BAX mediated release of Cytochrome c
- PMID: 33161099
- PMCID: PMC8418332
- DOI: 10.1016/j.joca.2020.08.014
Lysosomal dysfunction in osteoarthritis and aged cartilage triggers apoptosis in chondrocytes through BAX mediated release of Cytochrome c
Abstract
Objective: Lysosomes are the major catabolic organelle of the cell and regulate the macromolecular and organelle turnover and programmed cell death. Here, we investigated the lysosome dysfunction in cartilage and its role in chondrocytes apoptosis and the associated mechanism.
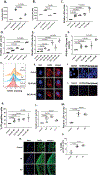
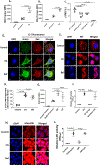
Design: Lysosomal acidification in Osteoarthritis (OA) and aged cartilage was determined by LysoSensor staining. Lysosomal function in chondrocytes was blocked by siRNA mediated depletion of Lysosomal Associated Membrane Protein 2 (LAMP2) or with lysosome inhibitors. Chondrocyte apoptosis was determined by LDH release, Caspase-3/7 activation, TUNEL and PI uptake assays. Loss of mitochondrial membrane potential (MMP/ΔΨM) and mitochondrial superoxide level was determined by JC-1 and MitoSOX staining, respectively. Colocalization of mitochondria with BCL2 associated X (BAX) and Cytochrome c was determined by immunostaining. Destabilization of medial meniscus (DMM) was performed to induce OA in mice.
Results: Lysosomal acidification was found to be significantly decreased in aged mouse and human and mouse OA cartilage which also showed increased chondrocyte apoptosis. Inhibition of lysosomal function resulted in increased oxidative stress, accumulation of dysfunctional mitochondria and apoptosis in chondrocytes in monolayer and in cartilage explant cultures. Depletion of LAMP2 expression or treatment of chondrocytes with lysosomal function inhibitors increased the expression and mitochondrial translocation of BAX leading to Cytochrome c release. Lysosomal dysfunction-induced apoptosis in chondrocytes was not blocked by antioxidants MitoTempo or Diphenyleneiodonium (DPI) but was abrogated by inhibiting BAX.
Conclusion: Lysosomal dysfunction induce apoptosis in chondrocytes through BAX-mediated mitochondrial damage and release of Cytochrome c. Our data points to lysosomal function restoration and/or BAX inhibition in chondrocytes as a therapeutic approach for OA.
Keywords: Ageing; LAMP1; LAMP2; Lysosomes; Mitochondrial dysfunction; Redox.
Copyright © 2020 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures




References
-
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med 2013; 19: 983–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

