Inflammation-Associated Senescence Promotes Helicobacter pylori-Induced Atrophic Gastritis
- PMID: 33161156
- PMCID: PMC7859172
- DOI: 10.1016/j.jcmgh.2020.10.015
Inflammation-Associated Senescence Promotes Helicobacter pylori-Induced Atrophic Gastritis
Erratum in
-
Correction.Cell Mol Gastroenterol Hepatol. 2024;17(3):515-516. doi: 10.1016/j.jcmgh.2023.11.010. Epub 2024 Jan 13. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38220484 Free PMC article. No abstract available.
Abstract
Background & aims: The association between cellular senescence and Helicobacter pylori-induced atrophic gastritis is not clear. Here, we explore the role of cellular senescence in H pylori-induced atrophic gastritis and the underlying mechanism.
Methods: C57BL/6J mice were infected with H pylori for biological and mechanistic studies in vivo. Gastric precancerous lesions from patients and mouse models were collected and analyzed using senescence-associated beta-galactosidase, Sudan Black B, and immunohistochemical staining to analyze senescent cells, signaling pathways, and H pylori infection. Chromatin immunoprecipitation, luciferase reporter assays, and other techniques were used to explore the underlying mechanism in vitro.
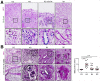
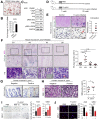
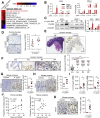
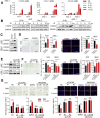
Results: Gastric mucosa atrophy was highly associated with cellular senescence. H pylori promoted gastric epithelial cell senescence in vitro and in vivo in a manner that depended on C-X-C motif chemokine receptor 2 (CXCR2) signaling. Interestingly, H pylori infection not only up-regulated the expression of CXCR2 ligands, C-X-C motif chemokine ligands 1 and 8, but also transcriptionally up-regulated the expression of CXCR2 via the nuclear factor-κB subunit 1 directly. In addition, CXCR2 formed a positive feedback loop with p53 to continually enhance senescence. Pharmaceutical inhibition of CXCR2 in an H pylori-infected mouse model attenuated mucosal senescence and atrophy, and delayed further precancerous lesion progression.
Conclusions: Our study showed a new mechanism of H pylori-induced atrophic gastritis through CXCR2-mediated cellular senescence. Inhibition of CXCR2 signaling is suggested as a potential preventive therapy for targeting H pylori-induced atrophic gastritis. GEO data set accession numbers: GSE47797 and GSE3556.
Keywords: C-X-C Motif Chemokine Receptor 2; H pylori; Mucosa Atrophy; Senescent Cell.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Senescence: A Novel Driver of Helicobacter pylori-Induced Gastric Atrophy.Cell Mol Gastroenterol Hepatol. 2021;11(3):887-888. doi: 10.1016/j.jcmgh.2020.12.005. Epub 2020 Dec 28. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33383028 Free PMC article. No abstract available.
References
-
- Correa P., Haenszel W., Cuello C., Zavala D., Fontham E., Zarama G., Tannenbaum S., Collazos T., Ruiz B. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–4740. - PubMed
-
- Correa P., Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. - PubMed
-
- Weck M.N., Brenner H. Association of Helicobacter pylori infection with chronic atrophic gastritis: meta-analyses according to type of disease definition. Int J Cancer. 2008;123:874–881. - PubMed
-
- Adamu M.A., Weck M.N., Rothenbacher D., Brenner H. Incidence and risk factors for the development of chronic atrophic gastritis: five year follow-up of a population-based cohort study. Int J Cancer. 2011;128:1652–1658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

