Dutch Oncology COVID-19 consortium: Outcome of COVID-19 in patients with cancer in a nationwide cohort study
- PMID: 33161241
- PMCID: PMC7540213
- DOI: 10.1016/j.ejca.2020.09.027
Dutch Oncology COVID-19 consortium: Outcome of COVID-19 in patients with cancer in a nationwide cohort study
Abstract
Aim of the study: Patients with cancer might have an increased risk for severe outcome of coronavirus disease 2019 (COVID-19). To identify risk factors associated with a worse outcome of COVID-19, a nationwide registry was developed for patients with cancer and COVID-19.
Methods: This observational cohort study has been designed as a quality of care registry and is executed by the Dutch Oncology COVID-19 Consortium (DOCC), a nationwide collaboration of oncology physicians in the Netherlands. A questionnaire has been developed to collect pseudonymised patient data on patients' characteristics, cancer diagnosis and treatment. All patients with COVID-19 and a cancer diagnosis or treatment in the past 5 years are eligible.
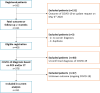
Results: Between March 27th and May 4th, 442 patients were registered. For this first analysis, 351 patients were included of whom 114 patients died. In multivariable analyses, age ≥65 years (p < 0.001), male gender (p = 0.035), prior or other malignancy (p = 0.045) and active diagnosis of haematological malignancy (p = 0.046) or lung cancer (p = 0.003) were independent risk factors for a fatal outcome of COVID-19. In a subgroup analysis of patients with active malignancy, the risk for a fatal outcome was mainly determined by tumour type (haematological malignancy or lung cancer) and age (≥65 years).
Conclusion: The findings in this registry indicate that patients with a haematological malignancy or lung cancer have an increased risk of a worse outcome of COVID-19. During the ongoing COVID-19 pandemic, these vulnerable patients should avoid exposure to severe acute respiratory syndrome coronavirus 2, whereas treatment adjustments and prioritising vaccination, when available, should also be considered.
Keywords: COVID-19; Cancer; Cancer treatment; Coronavirus; Pandemic.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement D.D. reports personal fees from speakers fee MSD, personal fees from speakers fee Roche, personal fees from speakers fee AstraZeneca, personal fees from speakers fee BMS, personal fees from speakers fee Novartis, personal fees from speakers fee Pfizer, outside the submitted work; H.W. reports honoraria from Astellas and Roche and travel expenses from Ipsen, outside the submitted work; K.S. reports personal fees and advisory role for Novartis, personal fees from Roche, personal fees and advisory role for MSD, advisory role BMS, advisory role Pierre Fabre, advisory role Abbvie, outside the submitted work; L.H. reports other from Boehringer Ingelheim, other from BMS, other from Roche Genentech, other from BMS, grants from Roche Genentech, grants from Boehringer Ingelheim, other from AstraZeneca, personal fees from Quadia, grants from Astra Zeneca, other from Eli Lilly, other from Roche Genentech, other from Pfizer, other from MSD, other from Takeda, non-financial support from AstraZeneca, non-financial support from Novartis, non-financial support from BMS, non-financial support from MSD/Merck, non-financial support from GSK, non-financial support from Takeda, non-financial support from Blueprint Medicines, non-financial support from Roche Genentech, other from Amgen, outside the submitted work; A.D. reports personal fees from Roche, personal fees from Eli Lily, personal fees from Boehringer Ingelheim, personal fees from Pfizer, personal fees from BMS, personal fees from Novartis, personal fees from Takeda, personal fees from Pharmamar, non-financial support from Abbvie, grants from BMS, grants from Amgen, outside the submitted work; A.V. reports advisory board of BMS, MSD, Merck, Pfizer, Ipsen, Eisai, Pierre Fabre, Roche, Novartis, Sanofi, outside the submitted work. All remaining authors declare no competing interests.
Figures

References
-
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;324(8):782–793. - PubMed
-
- World Health Organization. Coronavirus disease (COVID-19) outbreak 2020.
-
- World Health Organization. Coronavirus disease (COVID-2019) situation reports.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

