COVID-19 update: The race to therapeutic development
- PMID: 33161277
- PMCID: PMC7584885
- DOI: 10.1016/j.drup.2020.100733
COVID-19 update: The race to therapeutic development
Abstract
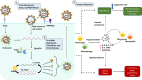
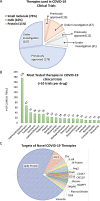
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), represents an unprecedented challenge to global public health. At the time of this review, COVID-19 has been diagnosed in over 40 million cases and associated with 1.1 million deaths worldwide. Current management strategies for COVID-19 are largely supportive, and while there are more than 2000 interventional clinical trials registered with the U.S. National Library of Medicine (clinicaltrials.gov), results that can clarify benefits and risks of candidate therapies are only gradually becoming available. We herein describe recent advances in understanding SARS-CoV-2 pathobiology and potential therapeutic targets that are involved in viral entry into host cells, viral spread in the body, and the subsequent COVID-19 progression. We highlight two major lines of therapeutic strategies for COVID-19 treatment: 1) repurposing the existing drugs for use in COVID-19 patients, such as antiviral medications (e.g., remdesivir) and immunomodulators (e.g., dexamethasone) which were previously approved for other disease conditions, and 2) novel biological products that are designed to target specific molecules that are involved in SARS-CoV-2 viral entry, including neutralizing antibodies against the spike protein of SARS-CoV-2, such as REGN-COV2 (an antibody cocktail), as well as recombinant human soluble ACE2 protein to counteract SARS-CoV-2 binding to the transmembrane ACE2 receptor in target cells. Finally, we discuss potential drug resistance mechanisms and provide thoughts regarding clinical trial design to address the diversity in COVID-19 clinical manifestation. Of note, preventive vaccines, cell and gene therapies are not within the scope of the current review.
Keywords: ACE2; Antivirals; COVID-19; Drug development; Existing drugs; Immunomodulators; Monoclonal antibodies; Repurposed use; SARS-CoV-2; Spike protein; Therapeutic targets; Virus life cycle.
Published by Elsevier Ltd.
Conflict of interest statement
The authors report no declarations of interest.
Figures
References
-
- 3DPrint . 2020. Prellis Biologics Pursues Bioprinted Lymph Nodes for Production of COVID-19 Antibodies.https://3dprint.com/267101/prellis-biologics-pursues-bioprinted-lymph-no... (07/09/2020)
-
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 - PMC - PubMed
-
- Artese A., Svicher V., Costa G., Salpini R., Di Maio V.C., Alkhatib M., Ambrosio F.A., Santoro M.M., Assaraf Y.G., Alcaro S., Ceccherini-Silberstein F. Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses. Drug Resist. Updates. 2020 p. 100721. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

