Mono-2-ethylhexyl phthalate drives progression of PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate cytotoxicity
- PMID: 33161305
- PMCID: PMC7649642
- DOI: 10.1016/j.redox.2020.101776
Mono-2-ethylhexyl phthalate drives progression of PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate cytotoxicity
Abstract
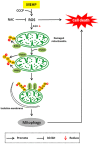
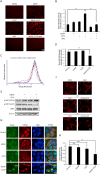
Phthalate ester plasticizers are used to improve the plasticity and strength of plastics. One of the most widely used and studied, di-2-ethylhexyl phthalate (DEHP), has been labeled as an endocrine disruptor. The major and toxic metabolic derivative of DEHP, mono-2-ethylhexyl phthalate (MEHP), is capable of interfering with mitochondrial function, but its mechanism of action on mitophagy remains elusive. Here, we report that MEHP exacerbates cytotoxicity by amplifying the PINK1-Parkin-mediated mitophagy pathway. First, MEHP exacerbated mitochondrial damage induced by low-dose CCCP via increased reactive oxygen species (ROS) production, decreased mitochondrial membrane potential (MMP), and enhanced fragmentation in mitochondria. Second, co-exposure to MEHP and CCCP ("MEHP-CCCP") induced robust mitophagy. Mechanistically, MEHP-CCCP stabilized PINK1, increased the level of phosphorylated ubiquitin (pSer 65-Ub), and led to Parkin mitochondrial translocation and activation. Third, MEHP-CCCP synergistically caused more cell death, while inhibition of mitophagy, either through chemical or gene silencing, reduced cell death. Finally and importantly, co-treatment with N-acetyl cysteine (NAC) completely counteracted the effects of MEHP-CCCP, suggesting that mitochondrial ROS played a vital role in this process. Our results link mitophagy and MEHP cytotoxicity, providing an insight into the potential roles of endocrine disrupting chemicals (EDCs) in human diseases such as Parkinson's disease.
Keywords: Cell death; Cytotoxicity; MEHP; Mitochondrial ROS; PINK1-Parkin-mediated mitophagy.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures






References
-
- Tubbs C.W., McDonough C.E. Reproductive impacts of endocrine-disrupting chemicals on wildlife species: implications for conservation of endangered species. Annual review of animal biosciences. 2018;6:287–304. - PubMed
-
- Rogers J.A., Metz L., Yong V.W. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013;53:421–430. - PubMed
-
- Guyot R., Chatonnet F., Gillet B., Hughes S., Flamant F. Toxicogenomic analysis of the ability of brominated flame retardants TBBPA and BDE-209 to disrupt thyroid hormone signaling in neural cells. Toxicology. 2014;325:125–132. - PubMed
-
- Kabir E.R., Rahman M.S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015;40:241–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

