Azaindole therapeutic agents
- PMID: 33161343
- PMCID: PMC7736151
- DOI: 10.1016/j.bmc.2020.115830
Azaindole therapeutic agents
Abstract
Azaindole structural framework is an integral part of several biologically active natural and synthetic organic molecules; and several FDA approved drugs for various diseases. In the last decade, quite a number of literature reports appeared describing the pharmacology, biological activity and therapeutic applications of a variety of azaindole molecules. This prompted the organic and medicinal chemistry community to develop novel synthetic methods for various azaindoles and test them for a bioactivity against a variety of biological targets. Herein, we have summarized the biological activity of therapeutically advanced clinical candidates and several preclinical candidate drugs that contain azaindole structural moiety.
Keywords: BMS626529; Fevipiprant and HIV treatment; Guitarrins; PLX4720; Pexidartinib.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CONFLICTS OF INTEREST
There are no conflicts to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


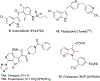

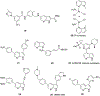


References
-
- Walker SR, Carter EJ, Huff BC, Morris JC, Variolins and Related Alkaloids, Chem. Rev, 109 (2009) 3080–3098. - PubMed
-
- Zhao S-B, Wang S, Luminescence and Reactivity of 7-Azaindole Derivatives and Complexes, Chem. Soc. Rev, 39 (2010) 3142–3156. - PubMed
-
- Merour J-Y, Joseph B, Synthesis and Reactivity of 7-Azaindoles (1H-pyrrolo[2,3-b]pyridines), Curr. Org. Chem, 5 (2001) 471–506.
-
- Echalier A, Bettayeb K, Ferandin Y, Lozach O, Clelment M, Valette A, Liger F, Marquet B, Morris JC, Endicott JA, Joseph B, Meijer L, Meriolins (3-(Pyrimidin-4-yl)-7-azaindoles): Synthesis, Kinase Inhibitory Activity, Cellular Effects, and Structure of a CDK2/Cyclin A/Meriolin Complex, J. Med. Chem, 51 (2008) 737–751. - PubMed
-
- Ermoli A, Bargiotti A, Brasca MG, Ciavolella A, Colombo N, Fachin G, Isacchi A, Menichincheri M, Molinari A, Montagnoli A, Pillan A, Rainoldi S, Sirtori FR, Sola F, Thieffine S, Tibolla M, Valsasina B, Volpi D, Santocanale C, Vanotti E, Cell Division Cycle 7 Kinase Inhibitors: 1H-Pyrrolo[2,3-b]pyridines, Synthesis and Structure-Activity Relationships, J. Med. Chem, 52 (2009) 4380–4390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

