Thioredoxin 1 is upregulated in the bone and bone marrow following experimental myocardial infarction: evidence for a remote organ response
- PMID: 33161477
- PMCID: PMC7847876
- DOI: 10.1007/s00418-020-01939-w
Thioredoxin 1 is upregulated in the bone and bone marrow following experimental myocardial infarction: evidence for a remote organ response
Abstract
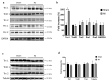
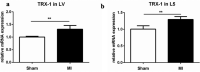
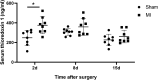
Ischemia and reperfusion events, such as myocardial infarction (MI), are reported to induce remote organ damage severely compromising patient outcomes. Tissue survival and functional restoration relies on the activation of endogenous redox regulatory systems such as the oxidoreductases of the thioredoxin (Trx) family. Trxs and peroxiredoxins (Prxs) are essential for the redox regulation of protein thiol groups and for the reduction of hydrogen peroxide, respectively. Here, we determined whether experimental MI induces changes in Trxs and Prxs in the heart as well as in secondary organs. Levels and localization of Trx1, TrxR1, Trx2, Prx1, and Prx2 were analyzed in the femur, vertebrae, and kidneys of rats following MI or sham surgery. Trx1 levels were significantly increased in the heart (P = 0.0017) and femur (P < 0.0001) of MI animals. In the femur and lumbar vertebrae, Trx1 upregulation was detected in bone-lining cells, osteoblasts, megakaryocytes, and other hematopoietic cells. Serum levels of Trx1 increased significantly 2 days after MI compared to sham animals (P = 0.0085). Differential regulation of Trx1 in the bone was also detected by immunohistochemistry 1 month after MI. N-Acetyl-cysteine treatment over a period of 1 month induced a significant reduction of Trx1 levels in the bone of MI rats compared to sham and to MI vehicle. This study provides first evidence that MI induces remote organ upregulation of the redox protein Trx1 in the bone, as a response to ischemia-reperfusion injury in the heart.
Keywords: Bone; Myocardial infarction; Oxidative stress; Peroxiredoxin; Remote organ response; Thioredoxin.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding this paper.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

