High-Sensitivity C-Reactive Protein is a Prognostic Biomarker of Six-Month Disability after Traumatic Brain Injury: Results from the TRACK-TBI Study
- PMID: 33161875
- PMCID: PMC7987360
- DOI: 10.1089/neu.2020.7177
High-Sensitivity C-Reactive Protein is a Prognostic Biomarker of Six-Month Disability after Traumatic Brain Injury: Results from the TRACK-TBI Study
Abstract
Systemic inflammation impacts outcome after traumatic brain injury (TBI), but most TBI biomarker studies have focused on brain-specific proteins. C-reactive protein (CRP) is a widely used biomarker of inflammation with potential as a prognostic biomarker after TBI. The
Keywords: biomarkers; head trauma; traumatic brain injury.
Conflict of interest statement
No competing financial interests exist.
Figures



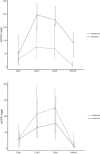
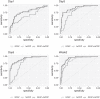
References
-
- Sandsmark, D.K. (2016). Clinical outcomes after traumatic brain injury. Curr. Neurol. Neurosci. Rep. 16, 52. - PubMed
-
- Foreman, B.P., Caesar, R.R., Parks, J., Madden, C., Gentilello, L.M., Shafi, S., Carlile, M.C., Harper, C.R., and Diaz-Arrastia, R.R. (2007). Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J. Trauma Acute Care Surg. 62, 946–950 - PubMed
-
- Diaz-Arrastia, R., Kochanek, P.M., Bergold, P., Kenney, K., Marx, C.E., Grimes, C.J.B., Loh, L.Y., Adam, L.G.E., Oskvig, D., and Curley, K.C. (2014). Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 31, 135–158 - PMC - PubMed
-
- Gabay, C., and Kushner, I. (1999). Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

