Risk scores for predicting early antiretroviral therapy mortality in sub-Saharan Africa to inform who needs intensification of care: a derivation and external validation cohort study
- PMID: 33161899
- PMCID: PMC7650165
- DOI: 10.1186/s12916-020-01775-8
Risk scores for predicting early antiretroviral therapy mortality in sub-Saharan Africa to inform who needs intensification of care: a derivation and external validation cohort study
Abstract
Background: Clinical scores to determine early (6-month) antiretroviral therapy (ART) mortality risk have not been developed for sub-Saharan Africa (SSA), home to 70% of people living with HIV. In the absence of validated scores, WHO eligibility criteria (EC) for ART care intensification are CD4 < 200/μL or WHO stage III/IV.
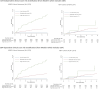
Methods: We used Botswana XPRES trial data for adult ART enrollees to develop CD4-independent and CD4-dependent multivariable prognostic models for 6-month mortality. Scores were derived by rescaling coefficients. Scores were developed using the first 50% of XPRES ART enrollees, and their accuracy validated internally and externally using South African TB Fast Track (TBFT) trial data. Predictive accuracy was compared between scores and WHO EC.
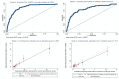
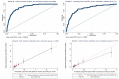
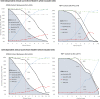
Results: Among 5553 XPRES enrollees, 2838 were included in the derivation dataset; 68% were female and 83 (3%) died by 6 months. Among 1077 TBFT ART enrollees, 55% were female and 6% died by 6 months. Factors predictive of 6-month mortality in the derivation dataset at p < 0.01 and selected for the CD4-independent score included male gender (2 points), ≥ 1 WHO tuberculosis symptom (2 points), WHO stage III/IV (2 points), severe anemia (hemoglobin < 8 g/dL) (3 points), and temperature > 37.5 °C (2 points). The same variables plus CD4 < 200/μL (1 point) were included in the CD4-dependent score. Among XPRES enrollees, a CD4-independent score of ≥ 4 would provide 86% sensitivity and 66% specificity, whereas WHO EC would provide 83% sensitivity and 58% specificity. If WHO stage alone was used, sensitivity was 48% and specificity 89%. Among TBFT enrollees, the CD4-independent score of ≥ 4 would provide 95% sensitivity and 27% specificity, whereas WHO EC would provide 100% sensitivity but 0% specificity. Accuracy was similar between CD4-independent and CD4-dependent scores. Categorizing CD4-independent scores into low (< 4), moderate (4-6), and high risk (≥ 7) gave 6-month mortality of 1%, 4%, and 17% for XPRES and 1%, 5%, and 30% for TBFT enrollees.
Conclusions: Sensitivity of the CD4-independent score was nearly twice that of WHO stage in predicting 6-month mortality and could be used in settings lacking CD4 testing to inform ART care intensification. The CD4-dependent score improved specificity versus WHO EC. Both scores should be considered for scale-up in SSA.
Trial registration: ClinicalTrials.gov NCT02538952.
Keywords: Acquired immuno-deficiency syndrome; Antiretroviral therapy; Clinical scores; HIV; Mortality; Predictive models.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- UNAIDS. AIDSinfo. Available at: http://aidsinfo.unaids.org/. Accessed 5 June 2020.
-
- Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, Bisson GP, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One. 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

