Assignment of opsonic values to pneumococcal reference serum 007sp and a second pneumococcal OPA calibration serum panel (Ewha QC sera panel B) for 11 serotypes
- PMID: 33162203
- PMCID: PMC7704772
- DOI: 10.1016/j.vaccine.2020.10.085
Assignment of opsonic values to pneumococcal reference serum 007sp and a second pneumococcal OPA calibration serum panel (Ewha QC sera panel B) for 11 serotypes
Abstract
Pneumococcal conjugate vaccines (PCVs) have been effective in reducing the disease burden caused by Streptococcus pneumoniae. The first licensed PCV (PCV7) was composed of capsular polysaccharides from seven serotypes. This was followed by PCV10, then PCV13, and currently there are a number of higher valency vaccines in development. As part of licensure, new vaccine iterations require assessment of immunogenicity. Since some antibodies can be non-functional, measuring functional antibodies is desirable. To meet this need, opsonophagocytic assays (OPAs) have been developed. Previous studies have shown there can be significant variations in OPA results from different laboratories. We have previously shown that standardizing OPA data using reference serum 007sp can decrease this variation. To extend this approach to additional serotypes, a panel of sera was tested by five laboratories using a multiplexed OPA for serotypes 2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20B, 22F, and 33F. Each sample was tested in five runs with 007sp tested three times in each run. Results were analyzed using a mixed effects ANOVA model. Standardization of the results significantly decreased the inter-laboratory variation for some serotypes. For serotypes 2, 8, and 11A, the variability was reduced by 40%, 45%, and 40%, respectively. For serotypes 12F, 17F, and 20B, the reductions were more modest (14%, 19%, and 24%, respectively). Standardization had little effect for the remaining serotypes. In many cases, the impact of normalization was blunted by the results from five sera that were collected after an extended post-vaccination interval. We have previously reported consensus values for 007sp for 13 serotypes, as well as the creation of a calibration serum panel ("Ewha Panel A"). Here, we report consensus values for 11 additional serotypes for 007sp and the creation of a second serum panel ("Ewha Panel B"). These consensus values will facilitate the development of next-generation PCVs.
Keywords: 007sp; Opsonophagocytic assay; Pneumococcus; Quality control sera; Standardization; Vaccines.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
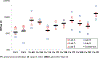



References
-
- Winkelstein JA. The role of complement in the host’s defense against Streptococcus pneumoniae. Rev Infect Dis. 1981;3:289–98. - PubMed
-
- Caro-Aguilar I, Indrawati L, Kaufhold RM, Gaunt C, Zhang Y, Nawrocki DK, et al. Immunogenicity differences of a 15-valent pneumococcal polysaccharide conjugate vaccine (PCV15) based on vaccine dose, route of immunization and mouse strain. Vaccine. 2017;35:865–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

