Complement activation, lipid metabolism, and mitochondrial injury: Converging pathways in age-related macular degeneration
- PMID: 33162377
- PMCID: PMC7767764
- DOI: 10.1016/j.redox.2020.101781
Complement activation, lipid metabolism, and mitochondrial injury: Converging pathways in age-related macular degeneration
Abstract
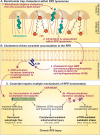
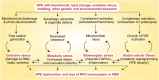
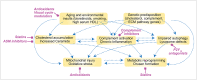
The retinal pigment epithelium (RPE) is the primary site of injury in non-neovascular age-related macular degeneration or dry AMD. Polymorphisms in genes that regulate complement activation and cholesterol metabolism are strongly associated with AMD, but the biology underlying disease-associated variants is not well understood. Here, we highlight recent studies that have used molecular, biochemical, and live-cell imaging methods to elucidate mechanisms by which aging-associated insults conspire with AMD genetic risk variants to tip the balance towards disease. We discuss how critical functions including lipid metabolism, autophagy, complement regulation, and mitochondrial dynamics are compromised in the RPE, and how a deeper understanding of these mechanisms has helped identify promising therapeutic targets to preserve RPE homeostasis in AMD.
Keywords: Autophagy; Ceramide; Cholesterol; Complement; Mitochondria; Retinal pigment epithelium.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Mitchell P., Liew G., Gopinath B., Wong T.Y. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. - PubMed

