Hypobaric hypoxia preconditioning protects against hypothalamic neuron apoptosis in heat-exposed rats by reversing hypothalamic overexpression of matrix metalloproteinase-9 and ischemia
- PMID: 33162790
- PMCID: PMC7645337
- DOI: 10.7150/ijms.47560
Hypobaric hypoxia preconditioning protects against hypothalamic neuron apoptosis in heat-exposed rats by reversing hypothalamic overexpression of matrix metalloproteinase-9 and ischemia
Abstract
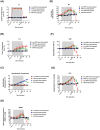
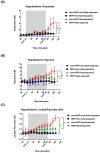
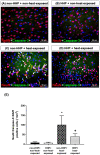
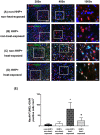
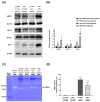
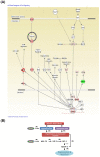
Background: Hypoxia-inducible factor-1α (HIF-1α), heat shock protein-72 (HSP-72), hemeoxygenase-1 (HO-1), and matrix metalloproteinase-9 (MMP-9) have been identified as potential therapeutic targets in the brain for cerebral ischemia. To elucidate their underlying mechanisms, we first aimed to ascertain whether these proteins participate in the pathogenesis of heat-induced ischemic damage to the hypothalamus of rats. Second, we investigated whether hypobaric hypoxia preconditioning (HHP) attenuates heat-induced hypothalamic ischemic/hypoxic injury by modulating these proteins in situ. Methods: Anesthetized rats treated with or without HHP were subjected to heat stress. Hypothalamic ischemic/hypoxic damage was evaluated by measuring hypothalamic levels of cerebral blood flow (CBF), partial oxygen pressure (PO2), and hypothalamic temperature via an implanted probe. Hypothalamic apoptotic neurons were counted by measuring the number of NeuN/caspase-3/DAPI triple-stained cells. Hypothalamic protein expression of HIF-1α, HSP-72, HO-1, and MMP-9 was determined biochemically. Results: Before the start of the thermal experiments, rats were subjected to 5 hours of HHP (0.66 ATA or 18.3% O2) daily for 5 consecutive days per week for 2 weeks, which led to significant loss of body weight, reduced brown adipose tissue (BAT) wet weight and decreased body temperature. The animals were then subjected to thermal studies. Twenty minutes after heat stress, heat-exposed rats not treated with HHP displayed significantly higher core and hypothalamic temperatures, hypothalamic MMP-9 levels, and numbers of hypothalamic apoptotic neurons but significantly lower mean blood pressure, hypothalamic blood flow, and PO2 values than control rats not exposed to heat. In heat-exposed rats, HHP significantly increased the hypothalamic levels of HIF-1α, HSP-72, and HO-1 but significantly alleviated body and hypothalamic hyperthermia, hypotension, hypothalamic ischemia, hypoxia, neuronal apoptosis and degeneration. Conclusions: HHP may protect against hypothalamic ischemic/hypoxic injury and overexpression of MMP-9 by upregulating the hypothalamic expression of HIF-1α, HSP-72, and HO-1 in rats subjected to heatstroke.
Keywords: heatstroke; hypobaric hypoxia; hypothalamus; ischemic/hypoxic injury.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Singh LP, Kapoor M, Singh SB. Heat: not black, not white. It's gray!!! J Basic Clin Physiol Pharmacol. 2013;24:209–24. - PubMed
-
- Leon LR, Bouchama A. Heat stroke. Compr Physiol. 2015;5:611–47. - PubMed
-
- Chauhan NR, Kapoor M, Prabha Singh L. et al. Heat stress-induced neuroinflammation and aberration in monoamine levels in hypothalamus are associated with temperature dysregulation. Neuroscience. 2017;358:79–92. - PubMed
-
- QIAGEN. QIAGEN Redwood City, QIAGEN'S Ingenuity Analysis 2015. [cited 2015; Available from: https:// www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/]
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

