A high expression ratio of RhoA/RhoB is associated with the migratory and invasive properties of basal-like Breast Tumors
- PMID: 33162807
- PMCID: PMC7645338
- DOI: 10.7150/ijms.43101
A high expression ratio of RhoA/RhoB is associated with the migratory and invasive properties of basal-like Breast Tumors
Abstract
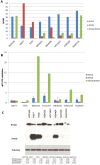
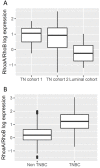
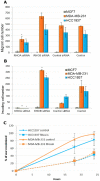
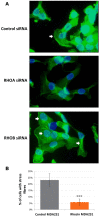
Basal-like breast cancer is among the most aggressive cancers and there is still no effective targeted treatment. In order to identify new therapeutic targets, we performed mRNA-Seq on eight breast cancer cell lines. Among the genes overexpressed in basal-like tumors, we focused on the RhoA and RhoB genes, which encode small GTPases known to play a role in the actin cytoskeleton, allowing cells to migrate. qRT-PCR and Western blotting were used for expression studies. Migratory and invasive properties were analysed by wound healing and Boyden chambers assays. Stress fibers formation was evaluated by fluorescent actin labeling. Rho siRNA, small inhibitor Rhosin treatment and BRCA1 transfection were performed to study the role of Rho and BRCA1 proteins. We showed that strong expression of RhoA and low expression of RhoB was associated with the basal-like subtype of breast cancer. Decreasing RhoA expression reduced the migratory and invasive capacities of basal-like cell lines, while decreasing RhoB expression increased these capacities. Rhosin, an inhibitor of RhoA, could also reduce the migration of basal-like cell lines. Rho proteins are involved in the formation of stress fibers, a conformation of the actin cytoskeleton found in migrating cells: inhibition of RhoA expression decreased the formation of these fibers. BRCA1, a gene frequently inactivated in basal-like tumors, appears to play a role in the differential expression of RhoA and RhoB in these tumors, as the restoration of BRCA1 expression in a BRCA1-mutated basal-like cell line decreased expression of RhoA and increased expression of RhoB, resulting in reduced migratory capacity. These results suggest Rho proteins as potential therapeutic targets for basal-like and BRCA1-mutated breast cancer, as migration and acquisition of mesenchymal properties are key functional pathways in these tumors with high metastatic potential.
Keywords: BRCA1; RhoA; RhoB; basal-like breast cancer; triple negative breast cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
The Role of RhoA, RhoB and RhoC GTPases in Cell Morphology, Proliferation and Migration in Human Cytomegalovirus (HCMV) Infected Glioblastoma Cells.Cell Physiol Biochem. 2016;38(1):94-109. doi: 10.1159/000438612. Epub 2016 Jan 8. Cell Physiol Biochem. 2016. PMID: 26741994
-
Antioxydation And Cell Migration Genes Are Identified as Potential Therapeutic Targets in Basal-Like and BRCA1 Mutated Breast Cancer Cell Lines.Int J Med Sci. 2018 Jan 1;15(1):46-58. doi: 10.7150/ijms.20508. eCollection 2018. Int J Med Sci. 2018. PMID: 29333087 Free PMC article.
-
A novel strategy for specifically down-regulating individual Rho GTPase activity in tumor cells.J Biol Chem. 2003 Nov 7;278(45):44617-25. doi: 10.1074/jbc.M308929200. Epub 2003 Aug 25. J Biol Chem. 2003. PMID: 12939257
-
RhoA, RhoB and RhoC have different roles in cancer cell migration.J Microsc. 2013 Sep;251(3):242-9. doi: 10.1111/jmi.12025. Epub 2013 Mar 12. J Microsc. 2013. PMID: 23488932 Review.
-
Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility.Exp Cell Res. 2004 Nov 15;301(1):43-9. doi: 10.1016/j.yexcr.2004.08.012. Exp Cell Res. 2004. PMID: 15501444 Review.
Cited by
-
Tissue-dependent mechanosensing by cells derived from human tumors.NPJ Biol Phys Mech. 2025;2(1):19. doi: 10.1038/s44341-025-00023-5. Epub 2025 Aug 6. NPJ Biol Phys Mech. 2025. PMID: 40786562 Free PMC article.
-
CDC45 promotes the stemness and metastasis in lung adenocarcinoma by affecting the cell cycle.J Transl Med. 2024 Apr 8;22(1):335. doi: 10.1186/s12967-024-05038-5. J Transl Med. 2024. PMID: 38589907 Free PMC article.
-
Scutellaria barbata D.Don extract regulates Ezrin-mediated triple negative breast cancer progress via suppressing the RhoA /ROCK1 signaling.Toxicol Res (Camb). 2024 Mar 21;13(2):tfae033. doi: 10.1093/toxres/tfae033. eCollection 2024 Apr. Toxicol Res (Camb). 2024. PMID: 38525246 Free PMC article.
-
Clinically Expired Platelet Concentrates as a Source of Extracellular Vesicles for Targeted Anti-Cancer Drug Delivery.Pharmaceutics. 2023 Mar 15;15(3):953. doi: 10.3390/pharmaceutics15030953. Pharmaceutics. 2023. PMID: 36986815 Free PMC article.
-
RHO GTPase-Related Long Noncoding RNAs in Human Cancers.Cancers (Basel). 2021 Oct 27;13(21):5386. doi: 10.3390/cancers13215386. Cancers (Basel). 2021. PMID: 34771549 Free PMC article. Review.
References
-
- Waddell N, Arnold J, Cocciardi S, da Silva L, Marsh A, Riley J. et al. Subtypes of familial breast tumours revealed by expression and copy number profiling. Breast Cancer Res Treat. 2010 Oct;123(3):661–77. - PubMed
-
- Valentin MD, da Silva SD, Privat M, Alaoui-Jamali M, Bignon Y-J. Molecular insights on basal-like breast cancer. Breast Cancer Res Treat. 2012 Jul;134(1):21–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

