Excitation of the Pre-frontal and Primary Visual Cortex in Response to Transcorneal Electrical Stimulation in Retinal Degeneration Mice
- PMID: 33162879
- PMCID: PMC7584448
- DOI: 10.3389/fnins.2020.572299
Excitation of the Pre-frontal and Primary Visual Cortex in Response to Transcorneal Electrical Stimulation in Retinal Degeneration Mice
Abstract
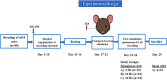
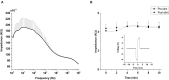
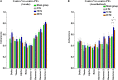
Retinal degeneration (rd) is one of the leading causes of blindness in the modern world today. Various strategies including electrical stimulation are being researched for the restoration of partial or complete vision. Previous studies have demonstrated that the effectiveness of electrical stimulation in somatosensory, frontal and visual cortices is dependent on stimulation parameters including stimulation frequency and brain states. The aim of the study is to investigate the effect of applying a prolonged electrical stimulation on the eye of rd mice with various stimulation frequencies, in awake and anesthetized brain states. We recorded spontaneous electrocorticogram (ECoG) neural activity in prefrontal cortex and primary visual cortex in a mouse model of retinitis pigmentosa (RP) after prolonged (5-day) transcorneal electrical stimulation (pTES) at various frequencies (2, 10, and 20 Hz). We evaluated the absolute power and coherence of spontaneous ECoG neural activities in contralateral primary visual cortex (contra Vcx) and contralateral pre-frontal cortex (contra PFx). Under the awake state, the absolute power of theta, alpha and beta oscillations in contra Vcx, at 10 Hz stimulation, was higher than in the sham group. Under the anesthetized state, the absolute power of medium-, high-, and ultra-high gamma oscillations in the contra PFx, at 2 Hz stimulation, was higher than in the sham group. We also observed that the ultra-high gamma band coherence in contra Vcx-contra PFx was higher than in the sham group, with both 10 and 20 Hz stimulation frequencies. Our results showed that pTES modulates rd brain oscillations in a frequency and brain state-dependent manner. These findings suggest that non-invasive electrical stimulation of retina changes patterns of neural oscillations in the brain circuitry. This also provides a starting point for investigating the sustained effect of electrical stimulation of the retina to brain activities.
Keywords: ECoG activity; pre-frontal cortex; primary visual cortex; retina; transcorneal electrical stimulation.
Copyright © 2020 Agadagba, Li and Chan.
Figures





References
-
- Agadagba S. K., Li X., Chan L. L. (2019). “Electroencephalogram power alterations in retinal degeneration mice after prolonged transcorneal electrical stimulation,” in Proceedings of the 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA.
LinkOut - more resources
Full Text Sources
Miscellaneous

