Evolution of Flight Muscle Contractility and Energetic Efficiency
- PMID: 33162892
- PMCID: PMC7581897
- DOI: 10.3389/fphys.2020.01038
Evolution of Flight Muscle Contractility and Energetic Efficiency
Abstract
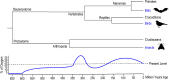
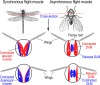
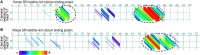
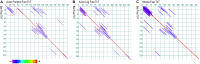
The powered flight of animals requires efficient and sustainable contractions of the wing muscles of various flying species. Despite their high degree of phylogenetic divergence, flight muscles in insects and vertebrates are striated muscles with similarly specialized sarcomeric structure and basic mechanisms of contraction and relaxation. Comparative studies examining flight muscles together with other striated muscles can provide valuable insights into the fundamental mechanisms of muscle contraction and energetic efficiency. Here, we conducted a literature review and data mining to investigate the independent emergence and evolution of flight muscles in insects, birds, and bats, and the likely molecular basis of their contractile features and energetic efficiency. Bird and bat flight muscles have different metabolic rates that reflect differences in energetic efficiencies while having similar contractile machinery that is under the selection of similar natural environments. The significantly lower efficiency of insect flight muscles along with minimized energy expenditure in Ca2+ handling is discussed as a potential mechanism to increase the efficiency of mammalian striated muscles. A better understanding of the molecular evolution of myofilament proteins in the context of physiological functions of invertebrate and vertebrate flight muscles can help explore novel approaches to enhance the performance and efficiency of skeletal and cardiac muscles for the improvement of human health.
Keywords: bat; bird; energetic efficiency; flight muscle; insect; molecular evolution; myofilament proteins; striated muscle.
Copyright © 2020 Cao and Jin.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

