Manipulation of Regulatory Dendritic Cells for Induction Transplantation Tolerance
- PMID: 33162996
- PMCID: PMC7591396
- DOI: 10.3389/fimmu.2020.582658
Manipulation of Regulatory Dendritic Cells for Induction Transplantation Tolerance
Abstract
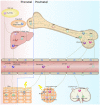
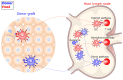
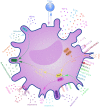
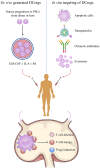
Current organ transplantation therapy is life-saving but accompanied by well-recognized side effects due to post-transplantation systematic immunosuppressive treatment. Dendritic cells (DCs) are central instigators and regulators of transplantation immunity and are responsible for balancing allograft rejection and tolerance. They are derived from monocyte-macrophage DC progenitors originating in the bone marrow and are classified into different subsets based on their developmental, phenotypical, and functional criteria. Functionally, DCs instigate allograft immunity by presenting donor antigens to alloreactive T cells via direct, indirect, and semidirect recognition pathways and provide essential signaling for alloreactive T cell activation via costimulatory molecules and pro-inflammatory cytokines. Regulatory DCs (DCregs) are characterized by a relatively low expression of major histocompatibility complex, costimulatory molecules, and altered cytokine production and exert their regulatory function through T cell anergy, T cell deletion, and regulatory T cell induction. In rodent transplantation studies, DCreg-based therapy, by in situ targeting or infusion of ex vivo generated DCregs, exhibits promising potential as a natural, well-tolerated, organ-specific therapeutic strategy for promoting lasting organ-specific transplantation tolerance. Recent early-phase studies of DCregs have begun to examine the safety and efficacy of DCreg-induced allograft tolerance in living-donor renal or liver transplantations. The present review summarizes the basic characteristics, function, and translation of DCregs in transplantation tolerance induction.
Keywords: T cell; allograft; dendritic cell; tolerance; transplantation.
Copyright © 2020 Que, Guo and Li.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

