A Review of the Progress and Challenges of Developing a Vaccine for COVID-19
- PMID: 33163000
- PMCID: PMC7591699
- DOI: 10.3389/fimmu.2020.585354
A Review of the Progress and Challenges of Developing a Vaccine for COVID-19
Abstract
A novel coronavirus, which has been designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first detected in December 2019 in Wuhan China and causes the highly infectious disease referred to as COVID-19. COVID-19 has now spread worldwide to become a global pandemic affecting over 24 million people as of August 26th, 2020 and claimed the life of more than 800,000 people worldwide. COVID-19 is asymptomatic for some individuals and for others it can cause symptoms ranging from flu-like to acute respiratory distress syndrome (ARDS), pneumonia and death. Although it is anticipated that an effective vaccine will be available to protect against COVID-19, at present the world is relying on social distancing and hygiene measures and repurposed drugs. There is a worldwide effort to develop an effective vaccine against SARS-CoV-2 and, as of late August 2020, there are 30 vaccines in clinical trials with over 200 in various stages of development. This review will focus on the eight vaccine candidates that entered Phase 1 clinical trials in mid-May, including AstraZeneca/Oxford's AZD1222, Moderna's mRNA-1273 and Sinovac's CoronaVac vaccines, which are currently in advanced stages of vaccine development. In addition to reviewing the different stages of vaccine development, vaccine platforms and vaccine candidates, this review also discusses the biological and immunological basis required of a SARS-CoV-2 vaccine, the importance of a collaborative international effort, the ethical implications of vaccine development, the efficacy needed for an immunogenic vaccine, vaccine coverage, the potential limitations and challenges of vaccine development. Although the demand for a vaccine far surpasses the production capacity, it will be beneficial to have a limited number of vaccines available for the more vulnerable population by the end of 2020 and for the rest of the global population by the end of 2021.
Keywords: COVID-19 pandemic; DNA vaccine; RNA vaccine; SARS-CoV-2; inactivated virus particle vaccine; neutralizing antibodies; non-replication viral vector vaccine; vaccine development.
Copyright © 2020 Sharma, Sultan, Ding and Triggle.
Figures

 symbol is a representation of the number of human subjects in trials.
symbol is a representation of the number of human subjects in trials.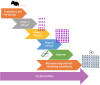
 symbol is a representation of the number of human subjects in trials.
symbol is a representation of the number of human subjects in trials.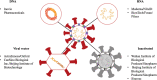
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

