Dynamical and allosteric regulation of photoprotection in light harvesting complex II
- PMID: 33163014
- PMCID: PMC7643867
- DOI: 10.1007/s11426-020-9771-2
Dynamical and allosteric regulation of photoprotection in light harvesting complex II
Abstract
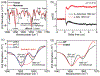
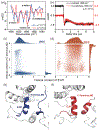
Major light-harvesting complex of photosystem II (LHCII) plays a dual role in light-harvesting and excited energy dissipation to protect photodamage from excess energy. The regulatory switch is induced by increased acidity, temperature or both. However, the molecular origin of the protein dynamics at the atomic level is still unknown. We carried out temperature-jump time-resolved infrared spectroscopy and molecular dynamics simulations to determine the energy quenching dynamics and conformational changes of LHCII trimers. We found that the spontaneous formation of a pair of local α-helices from the 310-helix E/loop and the C-terminal coil of the neighboring monomer, in response to the increased environmental temperature and/or acidity, induces a scissoring motion of transmembrane helices A and B, shifting the conformational equilibrium to a more open state, with an increased angle between the associated carotenoids. The dynamical allosteric conformation change leads to close contacts between the first excited state of carotenoid lutein 1 and chlorophyll pigments, facilitating the fluorescence quenching. Based on these results, we suggest a unified mechanism by which the LHCII trimer controls the dissipation of excess excited energy in response to increased temperature and acidity, as an intrinsic result of intense sun light in plant photosynthesis.
Keywords: FTIR; LHCII photoprotection; T-jump; conformational dynamics and allostery; excited energy transfer; fluorescence quenching; protein switch.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures





References
-
- Demmig-Adams B, Garab G, Adams W III, Govindjee. Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria. In: Govindjee, Sharkey TD, Eds. Advances in Photosynthesis and Respiration Including Bioenergy and Related Processes. Vol 40 The Netherlands: Springer Science & Business Media, 2014
-
- Rochaix JD. Annu Rev Plant Biol, 2014, 65: 287–309 - PubMed
-
- Ruban AV. FEBS Lett, 2018, 592: 3030–3039 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
