The role of lysosome in regulated necrosis
- PMID: 33163342
- PMCID: PMC7606114
- DOI: 10.1016/j.apsb.2020.07.003
The role of lysosome in regulated necrosis
Abstract
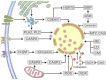
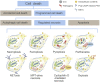
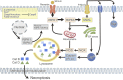
Lysosome is a ubiquitous acidic organelle fundamental for the turnover of unwanted cellular molecules, particles, and organelles. Currently, the pivotal role of lysosome in regulating cell death is drawing great attention. Over the past decades, we largely focused on how lysosome influences apoptosis and autophagic cell death. However, extensive studies showed that lysosome is also prerequisite for the execution of regulated necrosis (RN). Different types of RN have been uncovered, among which, necroptosis, ferroptosis, and pyroptosis are under the most intensive investigation. It becomes a hot topic nowadays to target RN as a therapeutic intervention, since it is important in many patho/physiological settings and contributing to numerous diseases. It is promising to target lysosome to control the occurrence of RN thus altering the outcomes of diseases. Therefore, we aim to give an introduction about the common factors influencing lysosomal stability and then summarize the current knowledge on the role of lysosome in the execution of RN, especially in that of necroptosis, ferroptosis, and pyroptosis.
Keywords: Ferroptosis; Lysosome; Necroptosis; Pyroptosis; Regulated necrosis.
© 2020 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures


References
-
- Appelqvist H., Wäster P., Kågedal K., Öllinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5:214–226. - PubMed
-
- Lawrence R.E., Zoncu R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat Cell Biol. 2019;21:133–142. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

