Androgen Receptor Signaling and Metabolic and Cellular Plasticity During Progression to Castration Resistant Prostate Cancer
- PMID: 33163409
- PMCID: PMC7581990
- DOI: 10.3389/fonc.2020.580617
Androgen Receptor Signaling and Metabolic and Cellular Plasticity During Progression to Castration Resistant Prostate Cancer
Abstract
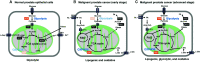
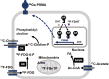
Metabolic reprogramming is associated with re/activation and antagonism of androgen receptor (AR) signaling that drives prostate cancer (PCa) progression to castration resistance, respectively. In particular, AR signaling influences the fates of citrate that uniquely characterizes normal and malignant prostatic metabolism (i.e., mitochondrial export and extracellular secretion in normal prostate, mitochondrial retention and oxidation to support oxidative phenotype of primary PCa, and extra-mitochondrial interconversion into acetyl-CoA for fatty acid synthesis and epigenetics in the advanced PCa). The emergence of castration-resistant PCa (CRPC) involves reactivation of AR signaling, which is then further targeted by androgen synthesis inhibitors (abiraterone) and AR-ligand inhibitors (enzalutamide, apalutamide, and daroglutamide). However, based on AR dependency, two distinct metabolic and cellular adaptations contribute to development of resistance to these agents and progression to aggressive and lethal disease, with the tumor ultimately becoming highly glycolytic and with imaging by a tracer of tumor energetics, 18F-fluorodoxyglucose (18F-FDG). Another major resistance mechanism involves a lineage alteration into AR-indifferent carcinoma such a neuroendocrine which is diagnostically characterized by robust 18F-FDG uptake and loss of AR signaling. PCa is also characterized by metabolic alterations such as fatty acid and polyamine metabolism depending on AR signaling. In some cases, AR targeting induces rather than suppresses these alterations in cellular metabolism and energetics, which can be explored as therapeutic targets in lethal CRPC.
Keywords: aerobic glycolysis; androgen receptor; castration-resistant prostate cancer; fatty acid metabolism; metabolic reprogramming 18F-FDG; mitochondria; neuroendocrine.
Copyright © 2020 Uo, Sprenger and Plymate.
Figures


References
-
- Lodish HF, Berk A, Kaiser C, Krieger M, Bretscher A, Ploegh HL, et al. Mol Cell Biol (2016).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

