Randomized Control Trial of Postnatal rhIGF-1/rhIGFBP-3 Replacement in Preterm Infants: Post-hoc Analysis of Its Effect on Brain Injury
- PMID: 33163463
- PMCID: PMC7581737
- DOI: 10.3389/fped.2020.517207
Randomized Control Trial of Postnatal rhIGF-1/rhIGFBP-3 Replacement in Preterm Infants: Post-hoc Analysis of Its Effect on Brain Injury
Abstract
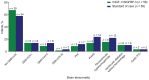
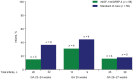
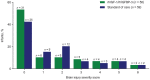
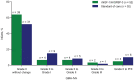
Background: Postnatal insulin-like growth factor-1 (IGF-1) replacement with recombinant human (rh)IGF-1 and IGF binding protein-3 (rhIGF-1/rhIGFBP-3) is being studied as a potential treatment to reduce comorbidities of prematurity. We have recently reported on a phase II, multicenter, randomized, controlled trial comparing postnatal rhIGF-1/rhIGFBP-3 replacement with standard of care (SOC) in extremely preterm infants (NCT01096784). Maximum severity of retinopathy of prematurity was the primary endpoint of the trial and presence of GMH-IVH/PHI one of the pre-specified secondary endpoints. Infants therefore received serial cranial ultrasound scans (CUS) between birth and term age. In this post-hoc analysis we present a detailed analysis of the CUS data of this trial and evaluate the effect of postnatal rhIGF-1/rhIGFBP-3 replacement on the incidence of different kinds of brain injury in extremely preterm infants. Methods: This report is an exploratory post-hoc analysis of a phase II trial in which infants <28 weeks gestational age were randomly allocated to rhIGF-1/rhIGFBP-3 or SOC. Serial cranial ultrasounds were performed between birth and term-equivalent age. Presence of germinal matrix hemorrhage and intraventricular hemorrhage (GMH-IVH), periventricular hemorrhagic infarction (PHI), post-hemorrhagic ventricular dilatation, and white matter injury (WMI) were scored by two independent masked readers. Results: The analysis included 117 infants; 58 received rhIGF-1/rhIGFBP-3 and 59 received SOC. A trend toward less grade II-III GMH-IVH and PHI was observed in treated infants vs. SOC. A subanalysis of infants without evidence of GMH-IVH at study entry (n = 104) showed reduced progression to GMH-IVH in treated infants (25.0% [13/52] vs. 40.4% [21/52]; not significant). No effects of rhIGF-1/rhIGFBP-3 on WMI were observed. Conclusion: The potential protective effect of rhIGF-1/rhIGFBP-3 on the occurrence of GMH-IVH/PHI appeared most pronounced in infants with no evidence of GMH-IVH at treatment start.
Keywords: brain injury; cerebral hemorrhage; extremely preterm; neonate; recombinant human IGF-1.
Copyright © 2020 Horsch, Parodi, Hallberg, Malova, Björkman-Burtscher, Hansen-Pupp, Marlow, Beardsall, Dunger, van Weissenbruch, Smith, Hamdani, Mangili, Barton, Ramenghi, Hellström, Ley and the ROPP-2008-01 Study Team.
Figures
References
-
- Ancel PY, Goffinet F, Kuhn P, Langer B, Matis J, Hernandorena X, et al. EPIPAGE-2 Writing Group. Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. (2015) 169:230–8. 10.1001/jamapediatrics.2014.3351 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

