Nonlinear Optical Microscopy: From Fundamentals to Applications in Live Bioimaging
- PMID: 33163482
- PMCID: PMC7581943
- DOI: 10.3389/fbioe.2020.585363
Nonlinear Optical Microscopy: From Fundamentals to Applications in Live Bioimaging
Abstract
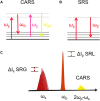
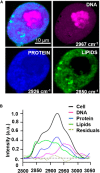
A recent challenge in the field of bioimaging is to image vital, thick, and complex tissues in real time and in non-invasive mode. Among the different tools available for diagnostics, nonlinear optical (NLO) multi-photon microscopy allows label-free non-destructive investigation of physio-pathological processes in live samples at sub-cellular spatial resolution, enabling to study the mechanisms underlying several cellular functions. In this review, we discuss the fundamentals of NLO microscopy and the techniques suitable for biological applications, such as two-photon excited fluorescence (TPEF), second and third harmonic generation (SHG-THG), and coherent Raman scattering (CRS). In addition, we present a few of the most recent examples of NLO imaging employed as a label-free diagnostic instrument to functionally monitor in vitro and in vivo vital biological specimens in their unperturbed state, highlighting the technological advantages of multi-modal, multi-photon NLO microscopy and the outstanding challenges in biomedical engineering applications.
Keywords: label-free microscopy; live imaging; nonlinear microscopy; stem cells; tissue engineering.
Copyright © 2020 Parodi, Jacchetti, Osellame, Cerullo, Polli and Raimondi.
Figures




References
-
- Barad Y., Eisenberg H., Horowitz M., Silberberg Y. (1997). Nonlinear scanning laser microscopy by third harmonic generation. Appl. Phys. Lett. 70 922–924. 10.1063/1.118442 - DOI
-
- Bloembergen N. (1982). Nonlinear optics and spectroscopy. Rev. Mod. Phys. 54 685–695.
Publication types
LinkOut - more resources
Full Text Sources

