Appeal and Use of Customizable E-cigarette Product Features in Adolescents
- PMID: 33163582
- PMCID: PMC7643857
- DOI: 10.18001/trs.4.2.5
Appeal and Use of Customizable E-cigarette Product Features in Adolescents
Abstract
Objectives: We evaluated the appeal and use of e-cigarette product features in a sample of high school students in Connecticut.
Methods: In 2015, we conducted school-wide surveys in 8 high schools and analyzed data from 1765 students (877 susceptible tobacco-naïve never-e-cigarette users, 315 susceptible tobacco-exposed never-e-cigarette users, and 656 current e-cigarette users). Students were asked: "What is cool about e-cigarettes?" Response options included e-liquid ingredients (eg, flavors, nicotine content, propylene glycol/vegetable glycerin ratio [ie, PG/VG), the heating source (eg, battery), and physical design (eg, color, shape, appearance). Students were asked: "How do you customize your e-cigarette?" (Change flavors, voltage, PG/VG levels, temperature, skin/tank color, other) and use of nicotine in the e-liquid (yes/no). Multivariable regression assessed associations between current e-cigarette users' customization and e-cigarette use frequency (#days/month).
Results: Flavors were the most appealing product feature among all groups. Among current users, using nicotine e-liquid was more prevalent than changing flavors (44.2% vs 29.6%). Using nicotine e-liquids and changing PG/vG ratios were associated with more frequent e-cigarette use among current users.
Conclusions: These findings can inform tobacco regulatory actions that put forth evidence-based recommendations to manufacture e-liquid flavors, nicotine concentrations and PG/VG ratios that prevent e-cigarette initiation and escalation of use.
Keywords: adolescent health; e-cigarettes; survey methods.
Conflict of interest statement
Conflict of Interest Statement None to report.
Figures
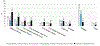
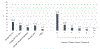

References
-
- US Food and Drug Administration. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. In US Department of Health and Human Services, ed. Vol 21 CFR 1100, 21 CFR 1140, 21 CFR 11432016:28973–29106. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
