Germ Cell Tumor Molecular Heterogeneity Revealed Through Analysis of Primary and Metastasis Pairs
- PMID: 33163850
- PMCID: PMC7608584
- DOI: 10.1200/PO.20.00166
Germ Cell Tumor Molecular Heterogeneity Revealed Through Analysis of Primary and Metastasis Pairs
Abstract
Purpose: Although primary germ cell tumors (GCTs) have been extensively characterized, molecular analysis of metastatic sites has been limited. We performed whole-exome sequencing and targeted next-generation sequencing on paired primary and metastatic GCT samples in a patient cohort enriched for cisplatin-resistant disease.
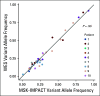
Patients and methods: Tissue sequencing was performed on 100 tumor specimens from 50 patients with metastatic GCT, and sequencing of plasma cell-free DNA was performed for a subset of patients.
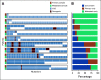
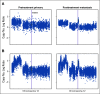
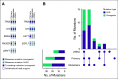
Results: The mutational landscape of primary and metastatic pairs from GCT patients was highly discordant (68% of all somatic mutations were discordant). Whereas genome duplication was common and highly concordant between primary and metastatic samples, only 25% of primary-metastasis pairs had ≥ 50% concordance at the level of DNA copy number alterations (CNAs). Evolutionary-based analyses revealed that most mutations arose after CNAs at the respective loci in both primary and metastatic samples, with oncogenic mutations enriched in the set of early-occurring mutations versus variants of unknown significance (VUSs). TP53 pathway alterations were identified in nine cisplatin-resistant patients and had the highest degree of concordance in primary and metastatic specimens, consistent with their association with this treatment-resistant phenotype.
Conclusion: Analysis of paired primary and metastatic GCT specimens revealed significant molecular heterogeneity for both CNAs and somatic mutations. Among loci demonstrating serial genetic evolution, most somatic mutations arose after CNAs, but oncogenic mutations were enriched in the set of early-occurring mutations as compared with VUSs. Alterations in TP53 were clonal when present and shared among primary-metastasis pairs.
© 2020 by American Society of Clinical Oncology.
Conflict of interest statement
Conflicts of Interest Statement:The authors have declared that no competing interests exist.Conflicts of Interest Statement:Authors’ disclosures of potential conflicts of interest and contributions are found at the end of this article.The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Michael L. ChengHonoraria: The Lynx Group (supported by Bristol Myers Squibb), WebMD (supported by AstraZeneca), PCME (supported by Merck and Lilly) Consulting or Advisory Role: AstraZeneca, Inivata Travel, Accommodations, Expenses: Daiichi Sankyo, Allergan, Sanofi, Natera, AstraZeneca, Guardant Health, WebMD, PCMEFrançois AudenetHonoraria: Nucleix Travel, Accommodations, Expenses: Ferring, IpsenEugene J. PietzakHonoraria: UpToDate Consulting or Advisory Role: Merck, Chugai PharmaSumit IsharwalOpen Payments Link: https://openpaymentsdata.cms.gov/physician/1232544Gopa IyerConsulting or Advisory Role: Bayer, Janssen, Mirati Therapeutics Research Funding: Mirati Therapeutics (Inst), Novartis (Inst), Debiopharm Group (Inst), Bayer (Inst)Samuel FuntStock and Other Ownership Interests: Kite Pharma, Urogen Pharma (I), Allogene Therapeutics, Neogene Therapeutics (I), Kronos Bio (I), Vida Ventures (I), Vaxigene Consulting or Advisory Role: AstraZeneca/MedImmune, Merck, Immunai Research Funding: Genentech (Inst), AstraZeneca (Inst), Decibel Therapeutics (Inst) Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca/MedImmuneDean F. BajorinHonoraria: Merck Sharp & Dohme Consulting or Advisory Role: Merck, Fidia Farmaceutici, Hoffman-La Roche, Dragonfly Therapeutics Research Funding: Novartis (Inst), Genentech (Inst), Merck (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Seattle Genetics/Astellas (Inst) Travel, Accommodations, Expenses: Genentech, MerckVictor E. ReuterConsulting or Advisory Role: Cepheid Uncompensated Relationships: PaigeAIGabriella JosephStock and Other Ownership Interests: AmgenMaria E. ArcilaHonoraria: Invivoscribe, Biocartis Consulting or Advisory Role: AstraZeneca Travel, Accommodations, Expenses: AstraZeneca, Invivoscribe, Raindance TechnologiesDana W.Y. TsuiHonoraria: Cowen, BofA Merrill Lynch Patents, Royalties, Other Intellectual Property: I am a co-inventor on a provisional patent application filed by Memorial Sloan Kettering Cancer CenterAhmet ZehirHonoraria: IlluminaMichael F. BergerConsulting or Advisory Role: Roche Research Funding: Grail Patents, Royalties, Other Intellectual Property: Provisional patent pending for “Systems and Methods for Detecting Cancer via cfDNA Screening”Eliezer Van AllenStock and Other Ownership Interests: Syapse, Tango Therapeutics, Genome Medical, Microsoft, Ervaxx Consulting or Advisory Role: Syapse, Roche, Third Rock Ventures, Takeda, Novartis, Genome Medical, InVitae, Illumina, Tango Therapeutics, Ervaxx, Janssen Speakers' Bureau: Illumina Research Funding: Bristol Myers Squibb, Novartis Patents, Royalties, Other Intellectual Property: Patent on discovery of retained intron as source of cancer neoantigens (Inst); patent on discovery of chromatin regulators as biomarkers of response to cancer immunotherapy (Inst); patent on clinical interpretation algorithms using cancer molecular data (Inst) Travel, Accommodations, Expenses: GenentechBarry S. TaylorConsulting or Advisory Role: Boehringer Ingelheim, Loxo Oncology at Lilly Research Funding: GenentechHikmat Al-AhmadieConsulting or Advisory Role: Bristol Myers Squibb, EMD Serono, AstraZeneca/MedImmune, Janssen BiotechDavid B. SolitStock and Other Ownership Interests: Loxo Consulting or Advisory Role: Pfizer, Loxo, Illumina, Vivideon Therapeutics, Lilly Oncology, QED Therapeutics, BridgeBio PharmaDarren R. FeldmanResearch Funding: Novartis, Seattle Genetics, Decibel Therapeutics (Inst), Astellas Pharma Other Relationship: UpToDate No other potential conflicts of interest were reported.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Hanna NH, Einhorn LH. Testicular cancer: Discoveries and updates. N Engl J Med. 2014;371:2005–2016. - PubMed
-
- International Germ Cell Cancer Collaborative Group International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

