Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells
- PMID: 33163980
- PMCID: PMC7598530
- DOI: 10.1016/j.xcrm.2020.100142
Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells
Abstract
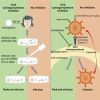
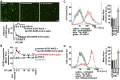
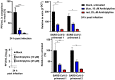
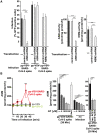
The acid sphingomyelinase/ceramide system plays an important role in bacterial and viral infections. Here, we report that either pharmacological inhibition of acid sphingomyelinase with amitriptyline, imipramine, fluoxetine, sertraline, escitalopram, or maprotiline or genetic downregulation of the enzyme prevents infection of cultured cells or freshy isolated human nasal epithelial cells with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or vesicular stomatitis virus (VSV) pseudoviral particles (pp-VSV) presenting SARS-CoV-2 spike protein (pp-VSV-SARS-CoV-2 spike), a bona fide system mimicking SARS-CoV-2 infection. Infection activates acid sphingomyelinase and triggers a release of ceramide on the cell surface. Neutralization or consumption of surface ceramide reduces infection with pp-VSV-SARS-CoV-2 spike. Treating volunteers with a low dose of amitriptyline prevents infection of freshly isolated nasal epithelial cells with pp-VSV-SARS-CoV-2 spike. The data justify clinical studies investigating whether amitriptyline, a safe drug used clinically for almost 60 years, or other antidepressants that functionally block acid sphingomyelinase prevent SARS-CoV-2 infection.
Keywords: SARS-CoV-2; acid sphingomyelinase; amitriptyline; antidepressants; ceramide; escitalopram; fluoxetine; human nasal epithelial cells; infection.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- World Health Organization Coronavirus disease (COVID-19): similarities and differences with influenza. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question...
-
- John Hopkins University Mortality analyses. https://coronavirus.jhu.edu/data/mortality
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

